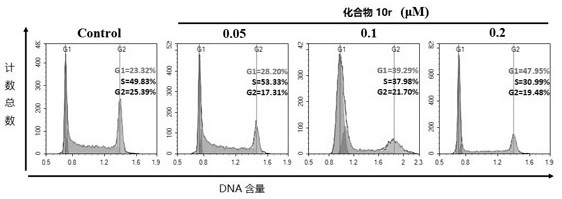
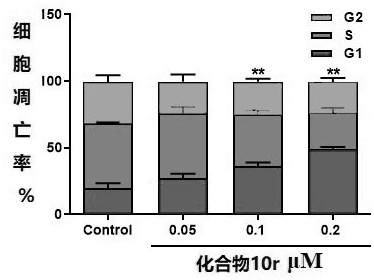
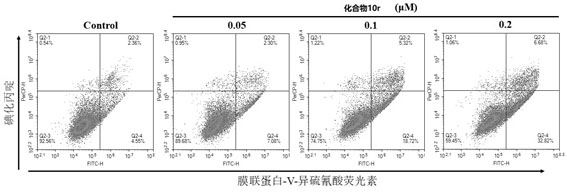
Locoagralan alcohol hydroxyl derivative as well as preparation method and application thereof
A technology of rocimilanol hydroxyl and derivatives, applied in organic chemistry methods, drug combinations, organic chemistry, etc., can solve problems such as side effects, effective dose and toxic dose side effects, difficulty in accepting chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation method of target compound 10a, comprises the following steps:
[0050] (1) Synthesis of compound 3
[0051]
[0052]Weigh 20g apigenin (compound 1, 0.0740mol) into a 500ml round-bottomed flask, add 51g anhydrous potassium carbonate (5.0eq) and 250ml acetone as a solvent to the flask, and slowly add 24.5 ml dimethyl sulfate under stirring (3.5eq), put the system into an oil bath and heated to reflux for 72h at 70°C. The reaction was followed by TLC. After the reaction was completed, it was cooled to room temperature and adjusted to pH=11 with ammonia water. The precipitate was removed by filtration, the filtrate was washed with a saturated saline solution, dried with anhydrous sodium sulfate, part of the solvent was spin-dried, silica gel was added to mix the sample, and purified using a flash silica gel column (chloroform:acetone=8:2) to obtain a pale yellow solid compound 2 ( 12g), 52.3% yield.
[0053] Weigh 700mg of compound 2 (2.2...
Embodiment 2
[0075] Example 2: Preparation of target compound 10b
[0076] The procedure was the same as that in Example 1, except that the benzoic acid was replaced by p-fluorobenzoic acid to prepare the target compound 10b in a yield of 58.9%.
[0077] 1-(p-Fluorobenzoyl-2-hydroxyacetyl)-rocmilanol (10b). White solid; mp 159-160°C; 1 H NMR (400MHz, CDCl 3 )δ:8.03–7.96(2H,m),7.16–7.05(9H,m),6.64(2H,d,J=9.0Hz),6.13(1H,d,J=1.9Hz),6.04(1H,d ,J=1.9Hz),5.94(1H,d,J=4.0 Hz),4.69(1H,d,J=16.0Hz),4.60(1H,d,J=16.0Hz),4.00(1H,dd,J =13.9, 6.1 Hz), 3.82 (3H, s), 3.81 (3H, s), 3.68 (3H, s), 2.88 (1H, td, J=13.9, 5.3 Hz), 2.35 (1H, ddd, J= 13.8, 6.1, 1.3Hz), 2.10 (1H, s); 13 C NMR (100MHz, CDCl 3 )δ: 166.5, 164.7, 163.7, 160.7, 158.6, 157.9, 138.2, 132.5, 132.4, 128.7, 128.7, 128.0, 128.0, 127.8, 127.8, 127.0, 126.3, 125.4, 115.6, 106.5, 112.6, 115.2 , 91.9, 88.2, 80.2, 60.9, 55.5, 55.5, 55.4, 55.0, 53.7, 35.5, 35.5; ESIMS m / z 637.3[M+Na] + ;HRESIMS m / z 649.1646[M+Cl] - (Calcd.forC 35 H 31 O 9...
Embodiment 3
[0078] Example 3: Preparation of target compound 10c
[0079] The steps were the same as those in Example 1, except that the benzoic acid was replaced with p-methoxybenzoic acid to prepare the target compound 10c with a yield of 55.9%.
[0080] 1-(p-Methoxybenzoyl-2-hydroxyacetyl)-rocmilanol (10c). White solid; mp 71-72°C; 1 H NMR (400MHz, CDCl 3 )δ: 7.94(2H,d,J=8.6Hz),7.14–7.06(7H,m), 6.90(2H,d,J=8.6Hz),6.64(2H,d,J=8.6Hz),6.15( 1H,d,J=1.9Hz),6.05(1H,d,J=1.9Hz),5.94(1H,dd,J=5.1,1.4Hz),4.67(1H,d,J=16.0Hz),4.57( 1H,d,J=16.0Hz), 4.01(1H,dd,J=13.7,6.1Hz), 3.87(3H,s), 3.82(3H,s), 3.80(3H,s), 3.68(3H,s) ), 2.87(1H,td,J=13.5,5.3Hz),2.36(1H,ddd,13.8,6.4,1.7Hz),2.12(1H,s); 13 C NMR (100MHz, CDCl 3 )δ: 166.8,165.4,163.7,163.6,160.7,158.6, 157.9,138.3,131.9,128.7,128.7,128.0,128.0,127.8,127.8,127.0,127.0,126.3, 121.5,106.2.6, , 91.8, 88.3, 80.1, 60.7, 55.5, 55.5, 55.5, 55.4, 55.0, 53.7, 35.5, 35.5; ESIMS m / z 649.3[M+Na] + ;HRESIMS m / z 661.1845[M +Cl] - (Calcd.for C 36 H 34 O ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com