Fluorescent probe and application thereof to detection of five aromatic pesticides
A fluorescent probe, fluorescence technology, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of fluorescent probe solution
[0028] Reagents: ThT (analytical grade) was purchased from Shanghai Aladdin Reagent Company, and the melon ring was taken from the Key Laboratory of Supramolecular Macrocycle Chemistry of Guizhou University (purity ≥98%).
[0029] Method: Accurately weigh 13.3 mg Q[8] and 3.2 mg ThT mixed with ultrapure water to prepare 100 mL, the molar concentration ratio is 1:1, and the molar concentration is 1×10 -4 mol / L fluorescent probe solution.
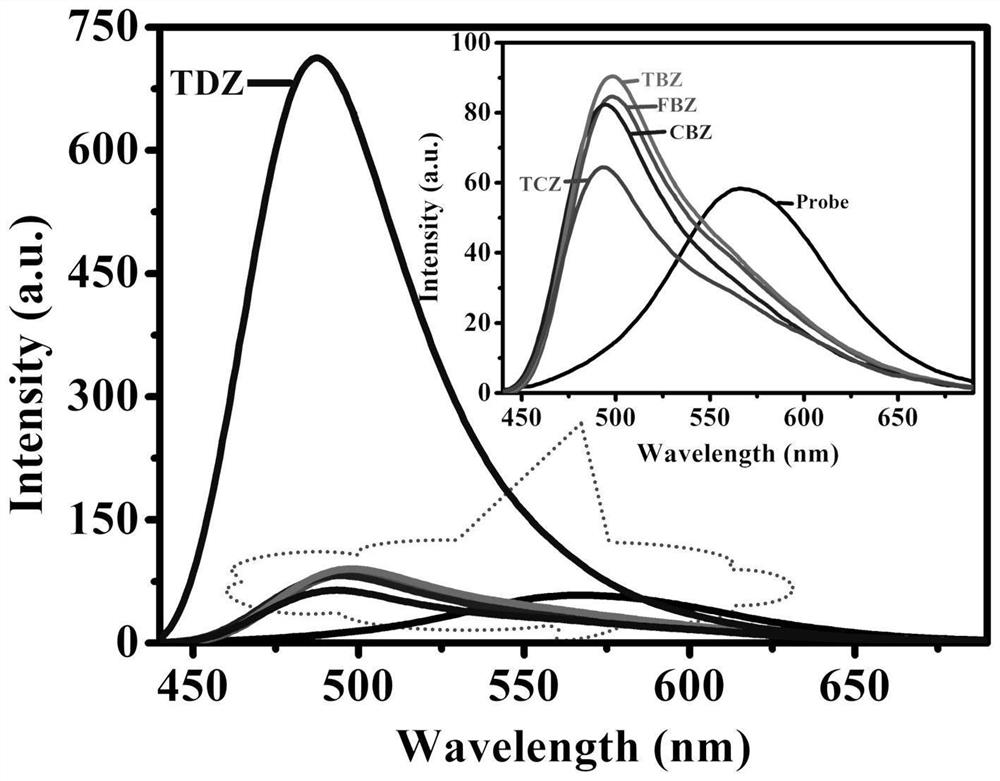
[0030] Among them, Q[8] is a supramolecular complex with a molar concentration ratio of 1:1 formed by the weak supramolecular interaction force of ion-dipole and hydrophobic interaction with ThT, which is referred to as a fluorescent probe, thereby causing the fluorescent red of ThT. Move to 567nm and enhance, when there are pesticide molecules, the pesticide molecules form a new ternary charge transfer complex with the fluorescent probe, called ternary complex for short, which ca...
Embodiment 2
[0031] Example 2: Establishment of a multi-target detection method
[0032] Reagents: FBZ, CBZ, TBZ, TDZ, TCZ (analytical grade) were purchased from Shanghai Aladdin Reagent Company.
[0033] Method: The concentrations of fluorescent probes and pesticide molecules were kept constant at 2×10 -5 mol / L, fixed excitation wavelength of 408 nm, excitation slit of 5 nm, emission slit of 5 nm, voltage of 600 V, and measured the fluorescence emission spectrum of the system.
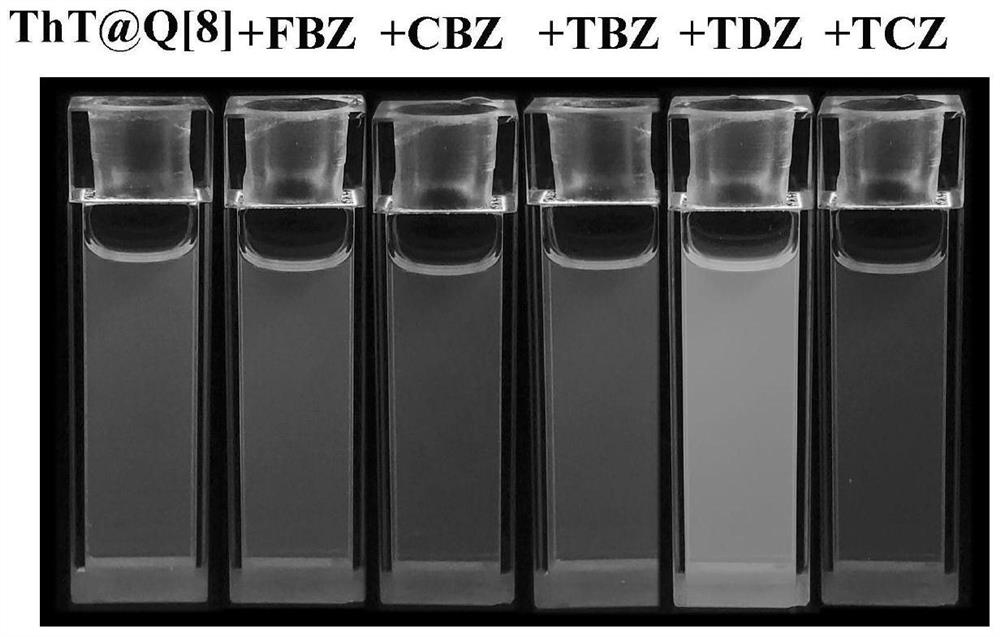
[0034] The aqueous solution of the fluorescent probe shows fluorescence at 567 nm. After adding aromatic pesticide molecules, FBZ and TBZ make the fluorescence emission peak of the system blue-shift from 567 nm to 498 nm, CBZ makes the fluorescence emission peak of the system blue-shift to 495 nm, and TCZ makes the fluorescence emission peak of the system blue-shifted. The emission peak was blue-shifted to 493 nm, and the intensity was enhanced by about 1-2 times. In addition, TDZ made the fluorescence blue-shift o...
Embodiment 3
[0038] Embodiment 3: Establishment of detection method
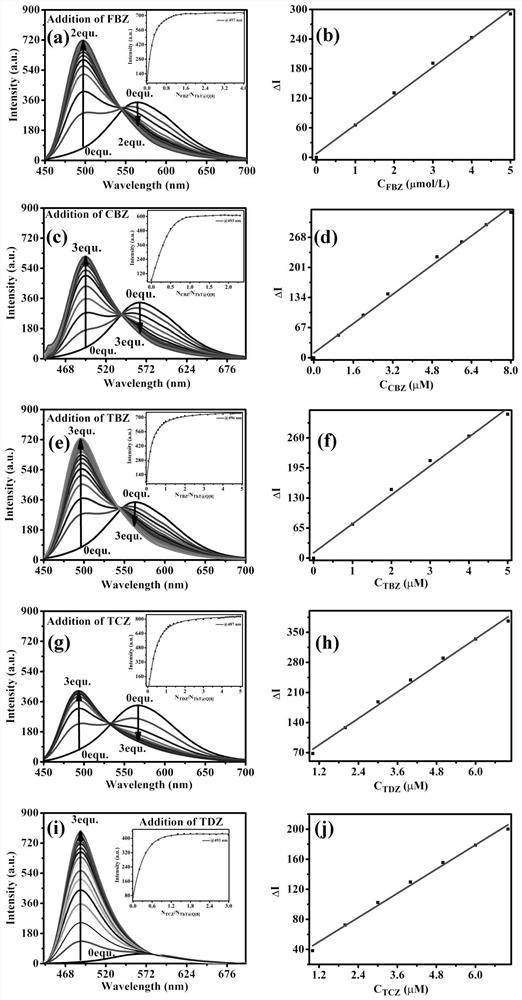
[0039] Method: The concentration of fluorescent probes was kept constant at 2 × 10 -5 mol / L, the concentration range of pesticide molecules is 2×10 -6 ~6×10 -5 mol / L, fixed excitation wavelength of 408 nm, slit 5 nm, and voltage of 600 V for fluorescence emission spectrum measurement, with the concentration of pesticide molecules as the abscissa, and the fluorescence emission spectrum intensity corresponding to 497 nm, 493 nm, 496 nm, 487 nm, and 493 nm as the ordinate to draw a standard curve .
[0040] According to the experimental results, the response linear ranges are: Maisuining and Thiabendazole: 0~5×10 -6 mol / L, carbendazim: 0~8×10 -6 mol / L, thidiazuron and tricyclazole: 0~7×10 -6 mol / L, the detection limits are: Mai Suining: 1.25×10 - 7 mol / L, carbendazim: 1.71×10 -7 mol / L, Thiabendazole: 1.17×10 -7 mol / L, Thidiazuron: 1.22×10 -7 mol / L, tricyclazole: 2.60×10 -7 mol / L, see attached for experimental res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com