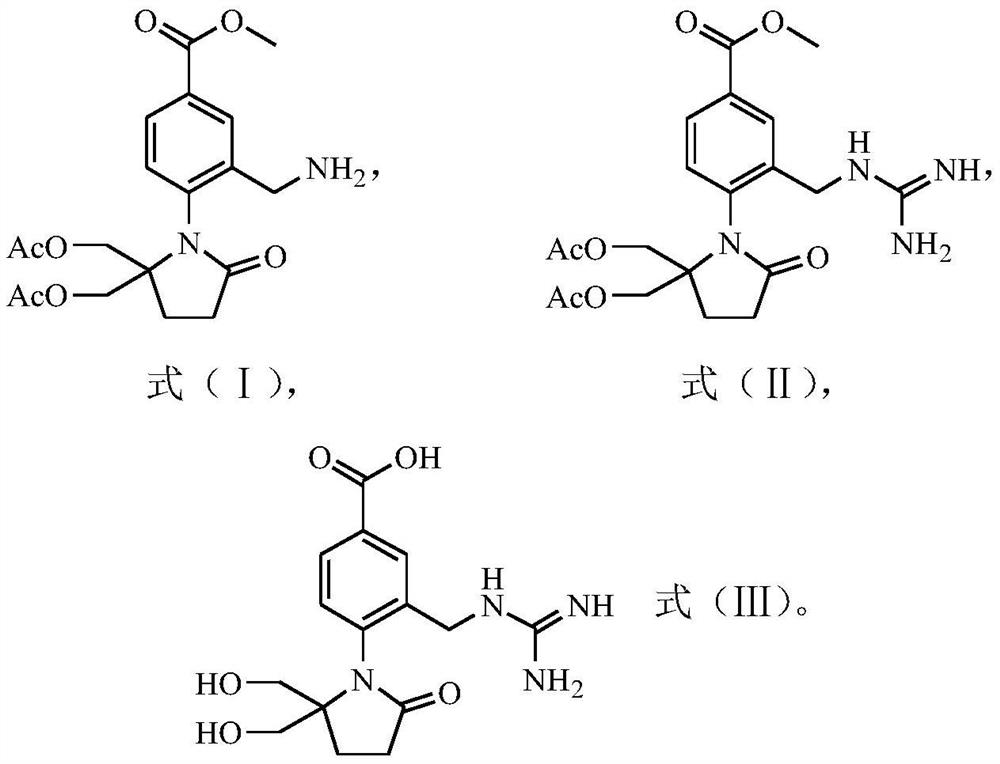
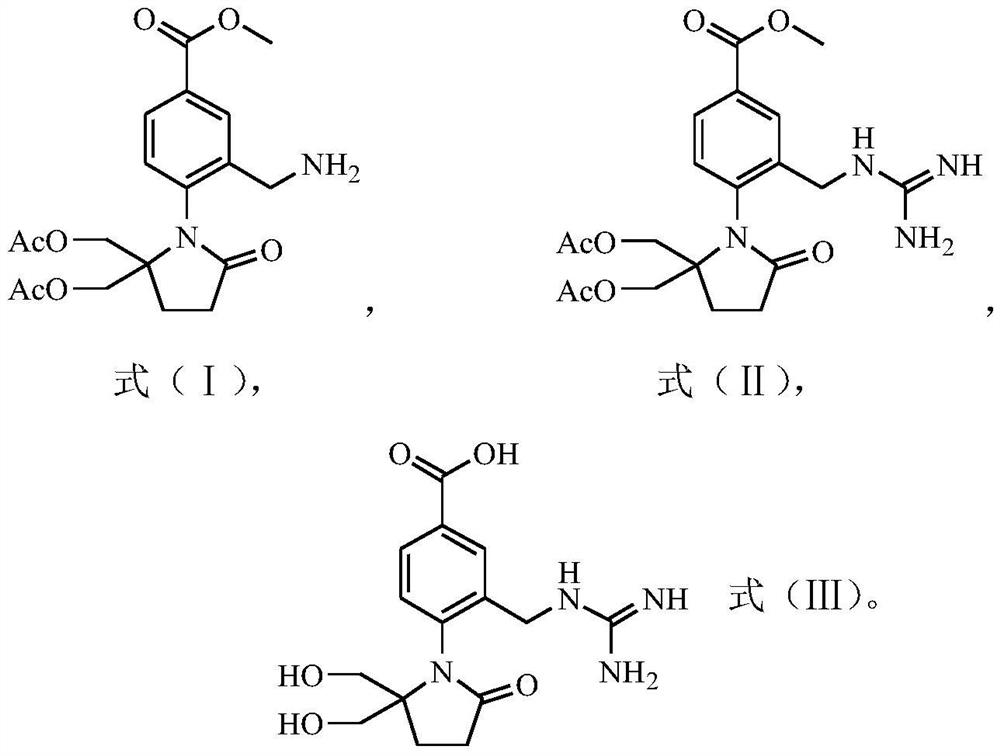
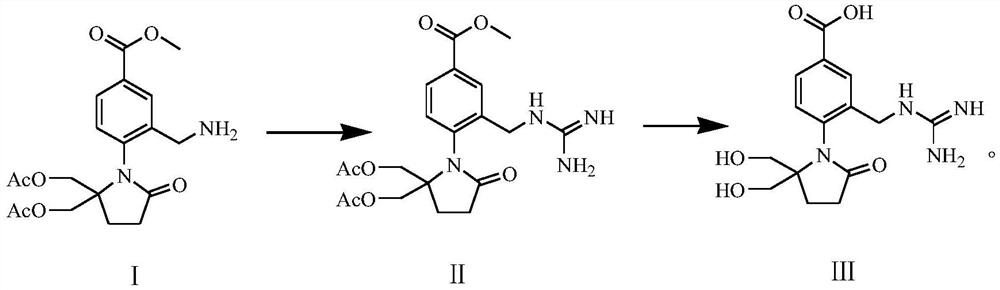
Preparation method of 4-(5, 5-dihydroxymethyl-2-oxopyrrolidinyl)-3-guanidino methylene benzoic acid
A technology of guanidinomethylene benzoic acid and oxypyrrolidinyl, which is applied in the field of synthesis of benzoic acid derivative drugs, can solve the problems of low yield of benzoic acid derivatives, unfavorable industrial production, low drug resistance of viruses, etc. Achieve the effects of short time period, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Formula (II) compound synthesis:
[0083] Weigh 1.14g (2.8mmol) of the compound of formula (I) and dissolve it in 20mL of 95% ethanol solution (ethanol / water (v / v)=95:5), then add 0.25mL of concentrated hydrochloric acid (HCl is 2.8mmol), After 0.40 g (9.5 mmol) of cyanamide, a cloudy mixed solution (pH 4.0) was obtained. The mixture was heated to reflux at a heating temperature of 78° C. until the solution became clear, and then stirred and heated to reflux for 1 h. The reaction solution was cooled to room temperature in a water bath at 20°C, and then concentrated hydrochloric acid was added to adjust the pH value to 1. Then 20 mL of water was added dropwise to the reaction solution. During the dropwise addition, crystals were continuously precipitated and crystallized at room temperature for 1 h; the temperature of the reaction solution was cooled to 3° C. in an ice-water bath, and then kept at 3° C. for crystallization for 1 h. Subsequent filtration obtained the fi...
Embodiment 2
[0087] The difference between this example and Example 1 is that when synthesizing the compound of formula (II), the amount of cyanamide added is 0.29g (7.0mmol).
[0088] When refluxing for 1 h, the reaction solution was taken for liquid chromatography detection, and the results showed that no compound of formula (I) was detected.
[0089] The reaction solution was treated according to the same process as in Example 1 to obtain a white solid (0.96 g, 79% yield) with a purity of 98.8%: MS (m / z) 435 (M+1).
[0090] The obtained compound of formula (II) was all used to prepare the compound of formula (III), and the preparation process was the same as in Example 1 to obtain a white solid (0.48g, 66% yield) with a purity of 99.1%: MS (m / z) 335 (M+1).
Embodiment 3
[0092] The difference between this example and Example 1 is that when synthesizing the compound of formula (II), the amount of cyanamide added is 0.35 g (8.4 mmol).
[0093] The reaction solution was treated according to the same process as in Example 1 to obtain a white solid (1.01 g, 83% yield) with a purity of 99.0%: MS (m / z) 435 (M+1).
[0094] The obtained compound of formula (II) was used to prepare the compound of formula (III), and the preparation process was the same as in Example 1 to obtain a white solid (0.59g, 77% yield) with a purity of 99.8%: MS (m / z) 335 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com