Indole diketone small molecule as well as preparation method and application thereof
A technology of indole dione, small molecule compound, applied in semiconductor/solid state device manufacturing, organic chemistry, electric solid device and other directions, can solve the problems of batch limitation, complex polymer synthesis, etc., achieve structure determination, synthesis method Simple, high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Synthesis of Indoledione Small Molecular Compounds of General Formula (I) [Compound (1)]
[0072] According to the following reaction formula, 30.0 mg of compound 1 (1 equiv.) and 8.2 mg of malononitrile (2.5 equiv.) were added to a side-necked flask, and nitrogen was pumped for three times. After the reaction was carried out for 2 h, the solvent in the system was evaporated by rotary evaporation, and the compound (1) was obtained by purification through a silica gel chromatography column (eluent is dichloromethane).
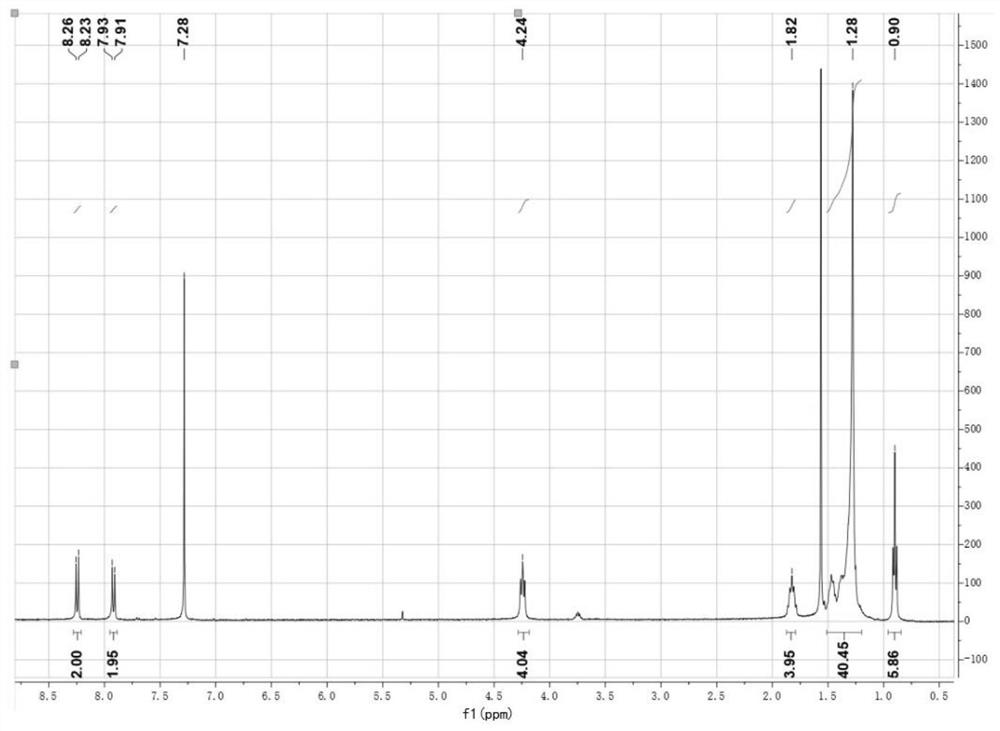
[0073] Compound (1) 1 H NMR and mass spectra, see figure 1 , Image 6 , and the NMR data are as follows:
[0074] 1 H NMR (400MHz, CDCl 3 ,300K),δ(ppm):8.25(d,2H),7.92(d,2H),4.24(t,4H),1.82(m,4H),1.23-1.50(m,40H),0.90(t, 6H).
[0075]
[0076] Wherein, compound 1 was prepared by the method described in the existing literature [Onwubiko A, Yue W, Jellett C, et al. Fusedelectron deficient semiconducting polymers for air stable electron t...
Embodiment 2
[0077] Example 2 Synthesis of Indoledione Small Molecular Compounds of General Formula (I) [Compound (2)]
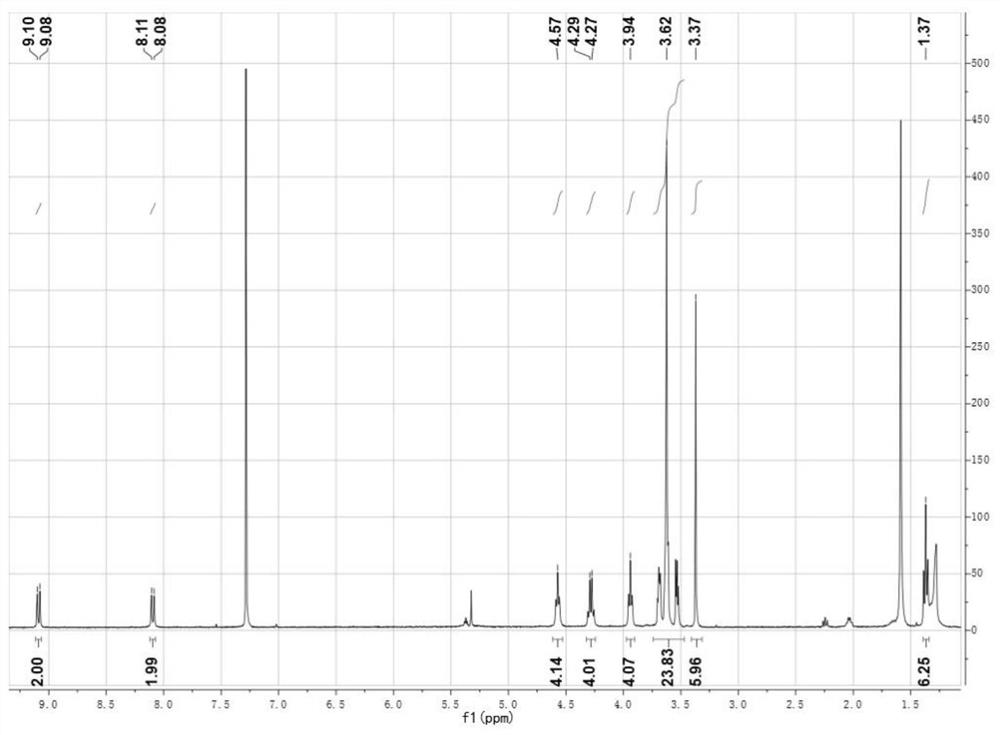
[0078] According to the following reaction formula, 30.0 mg of compound 2 (1 equiv.), 20.1 mg of 3-ethyl-2-thio-4-thiazolidinedione (2.5 equiv.) were added to a microwave tube, followed by 3 drops of triethylamine and 5 mL of ultra-dry chloroform was reacted at 60° C. for 1 h, the solvent in the system was evaporated by rotary evaporation, and the compound (2) was obtained by purification through a silica gel column (eluent: dichloromethane / methanol=100 / 2). Compound (2) 1 H NMR and mass spectra, see figure 2 , Figure 7 , and the NMR data are as follows:
[0079] 1 H NMR (400MHz, CDCl 3 ,300K),δ(ppm):9.09(d,2H),8.09(d,2H),4.57(t,4H),4.28(q,4H),3.94(t,4H),3.47-3.74(m, 24H), 3.37(s, 6H), 1.37(t, 6H).
[0080]
[0081] Among them, compound 2 was prepared by the method described in the existing literature (N-type Rigid Semiconducting Polymers Bearing Oligo (Ethyle...
Embodiment 3
[0082] Example 3 Synthesis of Indoledione Small Molecular Compounds of General Formula (I) [Compound (3)]
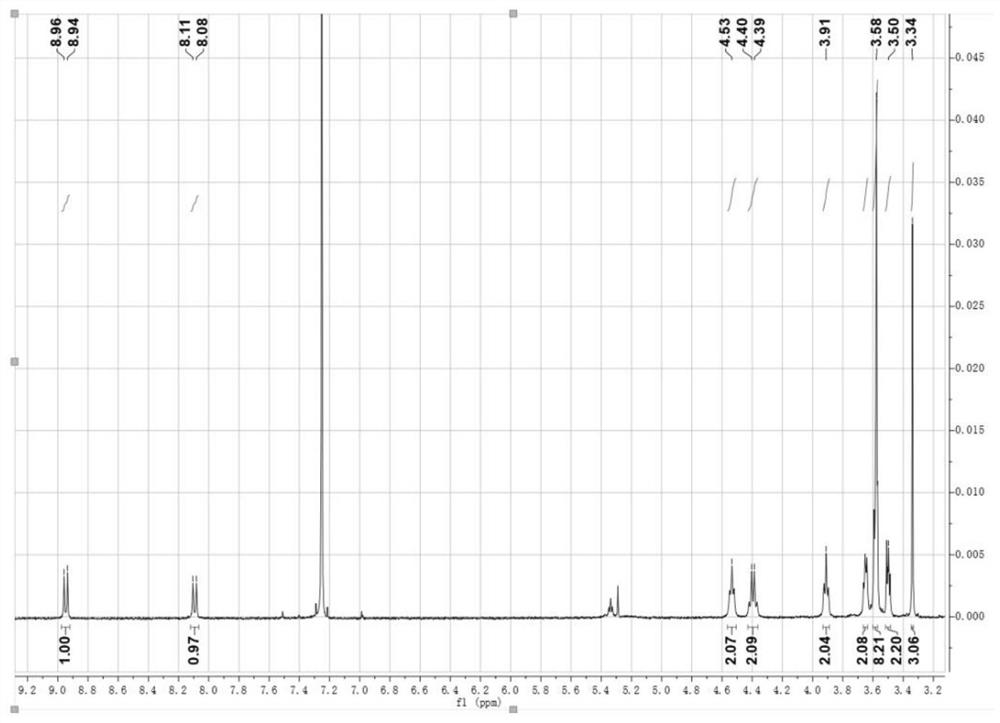
[0083] According to the following reaction formula, 30.0 mg of compound 2 (1 equivalent, the preparation method was the same as that of Example 2), 24.0 mg of 2-(3-ethyl-4-oxothiazolidine-2-ylidene)malononitrile (2.5 equivalents) ) into a microwave tube, then add 3 drops of triethylamine and 5 mL of ultra-dry chloroform, react at 60 °C for 1 h, evaporate the solvent in the system by rotary evaporation, and pass through a silica gel chromatography column (eluent is dichloromethane / methanol= 100 / 2) was purified to obtain compound (3). Compound (3) 1 H NMR chart see image 3 , and the NMR data are as follows:
[0084] 1 H NMR (400MHz, CDCl 3 ,300K),δ(ppm):8.95(d,2H),8.10(d,2H),4.53(t,4H),4.40(q,4H),3.91(t,4H),3.45-3.68(m, 24H), 3.34(s, 6H), 1.37(t, 6H).
[0085]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com