Nifedipine controlled release tablet and preparation method thereof
A technology of nifedipine and nifedipine, which is applied in the direction of medical formulas, medical preparations containing active ingredients, pill delivery, etc., can solve the problems of frequent doses, fluctuations in blood drug concentration, and fatigue, etc., so as to reduce the number of doses, Good internal and external correlation, the effect of improving safety
Pending Publication Date: 2022-07-01
HYBIO PHARMA
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] 1) Controlled-release preparations: The existing controlled-release preparations are mainly double-layer push-pull osmotic pump controlled-release preparations. Although this type of preparation can achieve zero-order release, after taking the drug, it will experience nearly two hours of release. The drug release starts after a lag time, and it takes several hours of drug release before the blood drug concentration can reach the effective therapeutic concentration. For patients who need to lower blood pressure in a short time, this type of preparation is relatively weak
[0006] 2) Sustained-release preparations: Although the time lag of sustained-release preparations is short and the drug action time is long, the drug cannot exhibit a good zero-order release, which is greatly affected by factors such as the pH value of the medium environment, gastrointestinal motility, and food. , poor correlation in vitro and in vivo
[0007] 3) Ordinary preparations: Ordinary preparations can easily cause large fluctuations in blood drug concentration, and the number of daily doses is large, which greatly weakens the safety, effectiveness and patient compliance of the drug
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0140]
Embodiment 2
[0142]
Embodiment 3
[0144]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
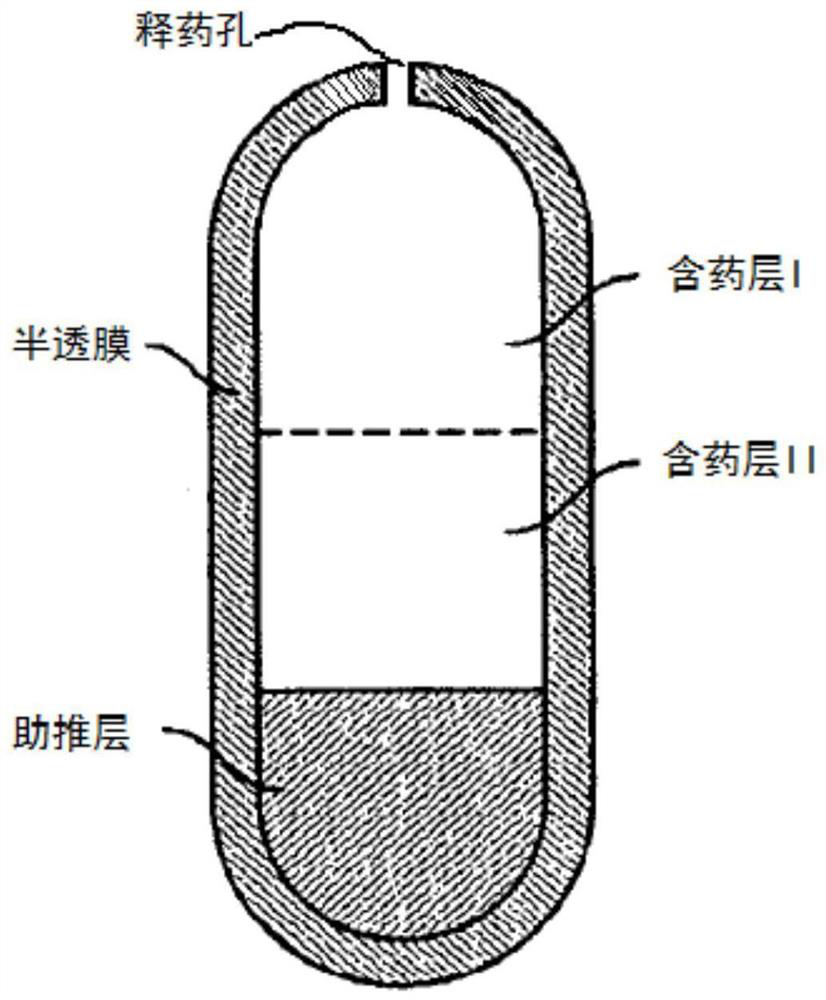
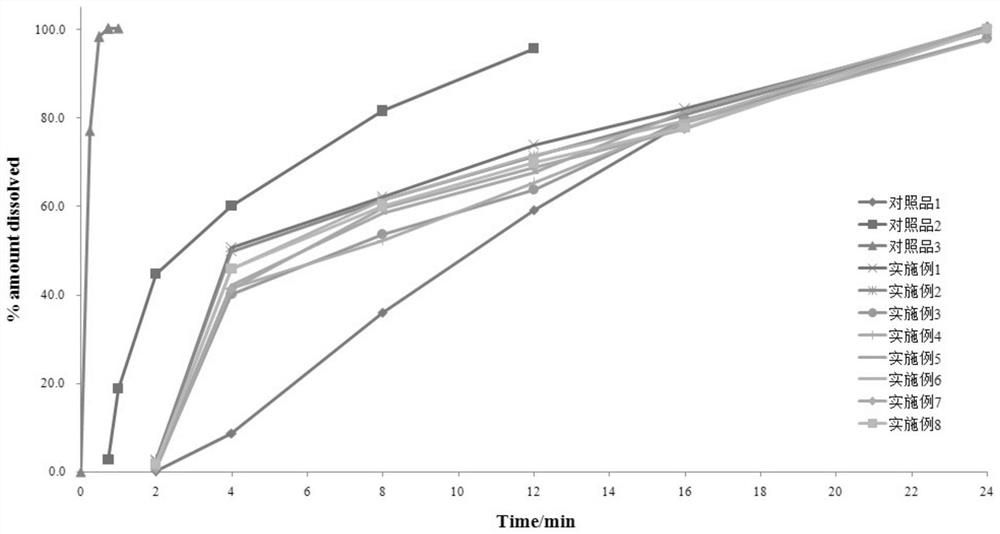
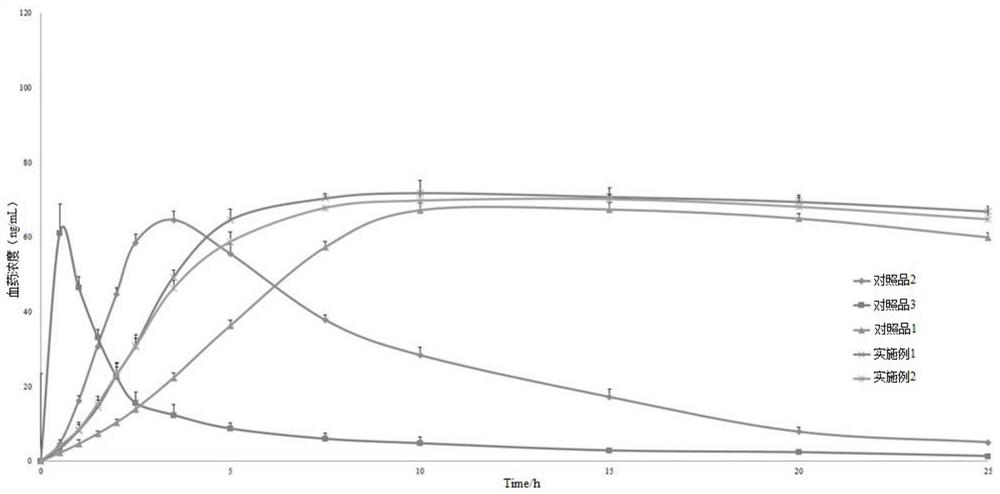
The invention discloses a nifedipine controlled release tablet and a preparation method thereof. The nifedipine controlled release tablet comprises a tablet core, a coating wrapping the tablet core and a gastric-soluble film coating wrapping the coating, the tablet core comprises a drug-containing layer I, a drug-containing layer II and a boosting layer which are arranged in sequence, and a drug release hole is formed in one side of the drug-containing layer I of the coating. A three-chamber push-pull osmotic pump controlled release technology is adopted, nifedipine is rapidly released within 4 hours after being taken through the drug-containing layer I and reaches the effective blood concentration, after the effective blood concentration is reached, the drug-containing layer II starts to release drugs, and the characteristic of zero-order release is maintained within the next 20 hours, so that the nifedipine sustained release tablet is prepared. And the blood concentration is kept in an effective blood concentration range.
Description
technical field [0001] The invention belongs to the field of pharmaceutical preparations, and relates to a nifedipine controlled-release tablet and a preparation method thereof. Background technique [0002] Nifedipine is a dihydropyridine calcium channel antagonist. This product can dilate the coronary arteries in the normal blood supply area and ischemic area at the same time, antagonize spontaneous or ergometrine-induced coronary artery spasm, and increase myocardial oxygenation in patients with coronary artery spasm. Deliver, relieve and prevent coronary spasm; can inhibit myocardial contraction, reduce myocardial metabolism, reduce myocardial oxygen consumption; dilate peripheral resistance blood vessels, reduce peripheral resistance, reduce systolic and diastolic blood pressure, and reduce cardiac afterload. It is commonly used clinically for various types of angina pectoris (especially variant angina pectoris), hypertension, refractory congestive heart failure and oth...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/36A61K9/24A61K31/4422A61P9/12A61P9/10A61P9/04
CPCA61K31/4422A61K9/209A61K9/2866A61P9/12A61P9/10A61P9/04A61K9/2095Y02A50/30
Inventor 吕志强张伟明唐洋明余品香
Owner HYBIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com