Preparation method of 3D printing hydrogel urethral stent
A 3D printing and hydrogel technology, applied in the field of 3D printing hydrogel urethral stents, can solve the problems of fast degradation rate, easy to be destroyed, unstable hydrogen bond cross-linking, etc., to achieve enhanced dispersion, good elasticity, enhanced The effect of tensile properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
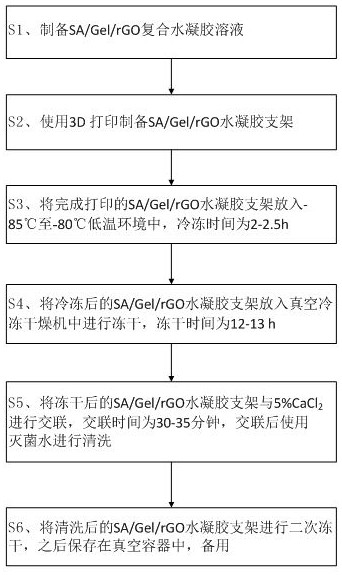
[0050] see Figure 1-9 As shown, a preparation method of a 3D printed hydrogel urethral stent includes the following steps:
[0051] S1, prepare SA / Gel / rGO composite hydrogel solution;
[0052] S2, using 3D printing to prepare SA / Gel / rGO hydrogel scaffolds;
[0053] S3, put the printed SA / Gel / rGO hydrogel scaffold in a low temperature environment of -85°C to -80°C, and the freezing time is 2-2.5h;
[0054] S4, put the frozen SA / Gel / rGO hydrogel scaffold into a vacuum freeze-drying machine for freeze-drying, and the freeze-drying time is 12-13 hours;
[0055] S5, lyophilized SA / Gel / rGO hydrogel scaffold with 5% CaCl 2 Carry out cross-linking, the cross-linking time is 30-35 minutes, and use sterilized water for cleaning after cross-linking;
[0056] S6, the washed SA / Gel / rGO hydrogel scaffold is lyophilized for a second time, and then stored in a vacuum container for later use.
[0057] Preparation of SA / Gel / rGO Composite Hydrogel
[0058] In step S1, the preparation of t...
Embodiment 2
[0079] On the basis of Example 1, the method further includes step: S7 , qualitatively analyzing the SA / Gel / rGO hydrogel scaffolds by means of a testing instrument.
[0080] In step S7, the testing instrument includes a Fourier transform infrared spectrometer, and Fourier transform infrared spectroscopy (FTIR) is used to characterize the functional groups of the SA / Gel / rGO composite hydrogel solution, and the prepared SA / Gel / rGO composite hydrogel is prepared. The gum solution was freeze-dried in a vacuum freeze dryer, after which it was finely ground with KBr (1:100) and compressed into flakes for infrared spectroscopy measurement in the range of 500-4000 cm -1 .
[0081] In step S7, the testing instrument further includes Raman spectroscopy, and qualitative analysis is performed on the incorporation of reduced graphene oxide in the SA / Gel / rGO composite hydrogel solution by Raman spectroscopy.
[0082] In step S7, the testing instrument further includes a scanning electron m...
Embodiment 3
[0093] On the basis of Example 2, the characterization of reduced graphene oxide (rGO) and the construction of composite hydrogels
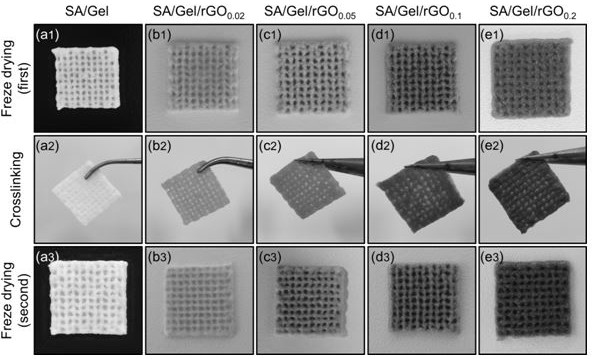
[0094] The monolayer rate of the reduced graphene oxide powder is increased by 80%, the diameter is 0.5-5 μm, and the thickness is 0.8-1.2 nm. Under SEM and TEM, rGO is a monolayer ( figure 2 a and b). Dispersion of rGO (1 mg mL -1 ) were diluted to concentrations of 0.02, 0.05, 0.1 and 0.2 mg mL -1 , they are all stable and uniform over several weeks, indicating that PVP can make the reduced graphene oxide well dispersed in water. As the concentration of reduced graphene oxide increases, the color of the solution changes from light to black, such as figure 2 c shown. To confirm the dispersion of reduced graphene oxide in the SA / Gel hydrogel composite solution, they were observed under an optical microscope. The results show that the reduced graphene oxide does not aggregate and is uniformly distributed (e.g. figure 2 d-g shown).
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com