Preparation method of eptifibatide impurity I
A technology for eptifibatide and impurities, which is applied in the field of preparation of eptifibatide impurity I, can solve the problems of no relevant literature reports on the preparation of impurity I, etc., and achieve high purity and yield, low cost, and easy industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a preparation method of eptifibatide impurity I. Those skilled in the art can learn from the content of this document and appropriately improve the process parameters to achieve. It should be particularly pointed out that all similar substitutions and modifications are obvious to those skilled in the art, and they are deemed to be included in the present invention. The method and application of the present invention have been described through the preferred embodiments, and it is obvious that relevant persons can make changes or appropriate changes and combinations of the methods and applications herein without departing from the content, spirit and scope of the present invention to realize and apply the present invention. Invention technology.
[0042] The test materials used in the present invention are all common commercial products and can be purchased in the market.
[0043] The invention provides a method for the preparation of eptifibatid...
Embodiment 1
[0062] Example 1 Synthesis of Compound 1
[0063] 2-Cl-Trt resin (85.7 g, Sub=1.4 mmol / g) was weighed into the solid-phase reaction column, washed twice with DMF, swollen with DMF for 30 minutes, and the solution was removed. Weigh Fmoc-Pro-OH 81.0g (240mmol), DIPEA125.7mL (480mmol) and 300ml DMF, add it to the reaction column, react at room temperature for 2 hours, then add 50mL methanol, react at room temperature for 1 hour, extract the solution, DMF Wash three times. A 20% piperidine solution was added to remove the Fmoc protecting group, the reaction was stirred at room temperature for 5 minutes, the solution was removed, the operation was repeated once, and the DMF was washed six times.
[0064] Weigh Boc-Trp(Boc)-OH 97.1 g (240 mmol), PyBOP 124.9 g (240 mmol), HOBt 38.9 g (288 mmol) and 300 ml DMF, slowly add DIPEA 125.7 mL (480 mmol) under ice bath for activation, then add the solution to In the reaction column, the reaction was carried out at room temperature for 2 h...
Embodiment 2
[0066] Example 2 Synthesis of Compound 2
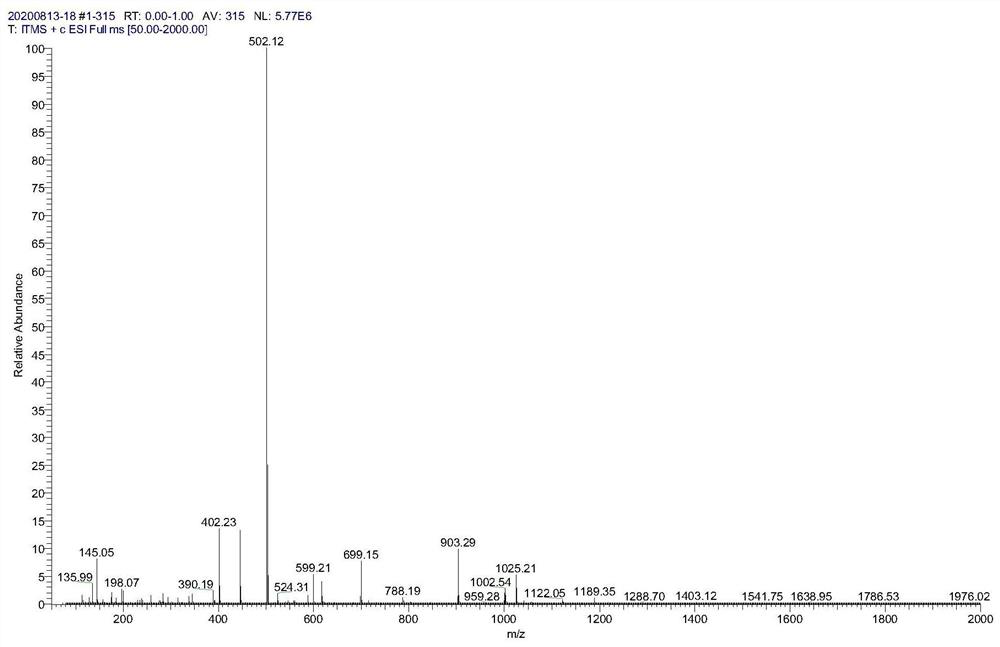
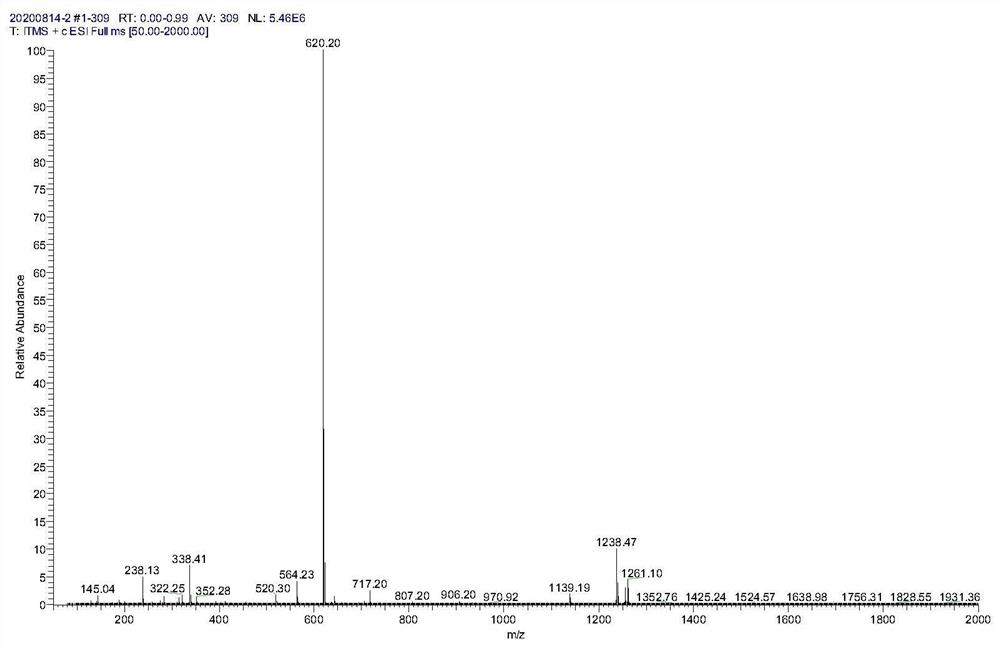
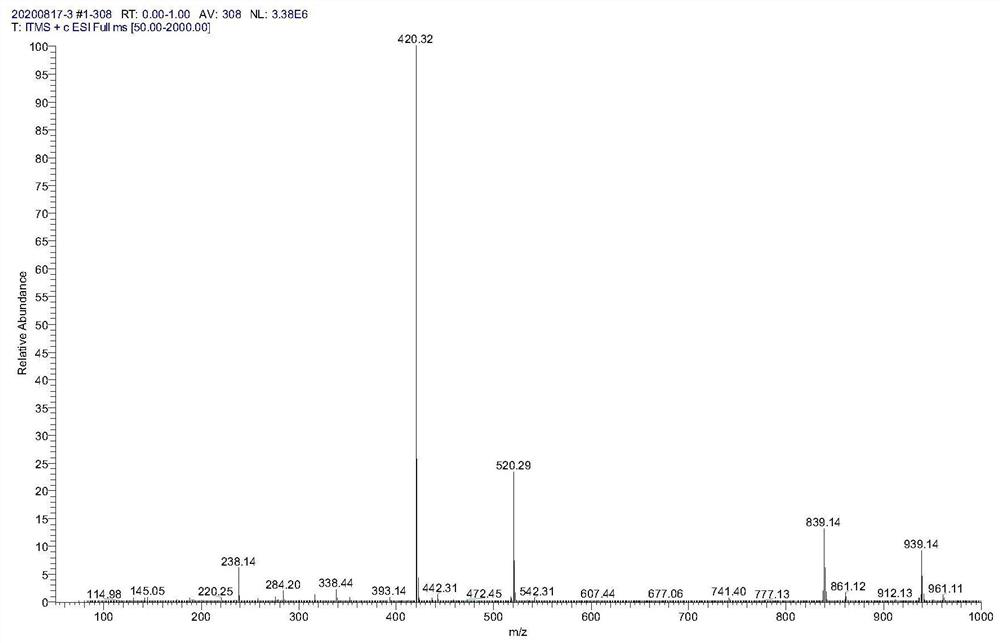
[0067] Weigh compound 1 (59.6g, 118mmol), 23.65g (178mmol) of 2-bromoacetophenone and 600ml of ethyl acetate into a glass bottle, add 75ml of triethylamine (535mmol) under ice bath, react at room temperature overnight, and monitor by TLC reaction. After the completion of the reaction of compound 1, it was washed with 10% citric acid solution, saturated sodium bicarbonate solution and saturated sodium chloride solution in turn, and the organic phase was concentrated to dryness under reduced pressure, and purified by silica gel column chromatography to obtain compound 2 (59 g). Mass spectrometry see figure figure 2 , which is consistent with the theory.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com