Influenza A virus detection kit and detection method thereof
A type of influenza A virus and detection kit technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganism measurement/inspection, etc., can solve the problems of time-consuming cost, lack of sensitivity and specificity of detection, etc., to achieve The effect of shortening the detection time, avoiding false positive results, and improving the detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Using the principle of Taqman fluorescence quantitative PCR, specific primers for influenza A virus are designed to amplify the specific nucleic acid sequence of influenza A virus, and Taqman probes are designed for the specific sequence of influenza A virus, located between the upstream and downstream primers. The probe for influenza A virus sequence is labeled with a fluorescent reporter group at the 5' end and a non-fluorescent quencher group at the 3' end. When the probe is intact, the fluorescence energy emitted by the reporter group is absorbed by the quencher group, and the instrument cannot detect the signal. With the progress of PCR, Taq enzyme encounters the probe bound to the template during the chain extension process, and its 5′→3′ exonuclease activity will cut off the probe, and the reporter group is far away from the quencher group, which The energy cannot be absorbed, i.e. a fluorescent signal is generated.
[0112] Ⅰ. Nucleic acid extraction of samples...
Embodiment 2
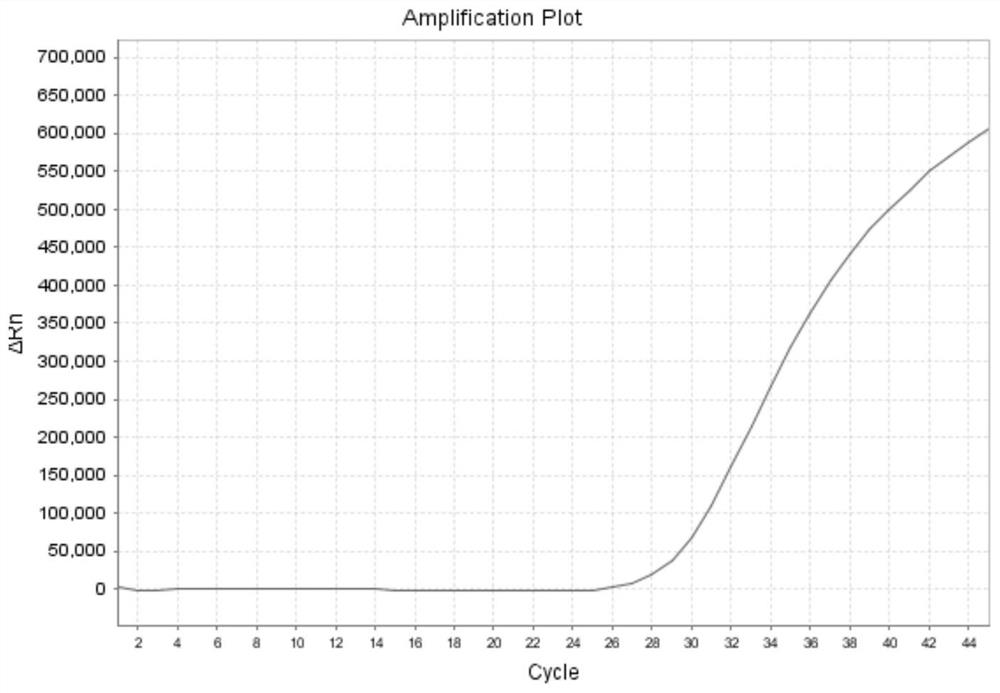
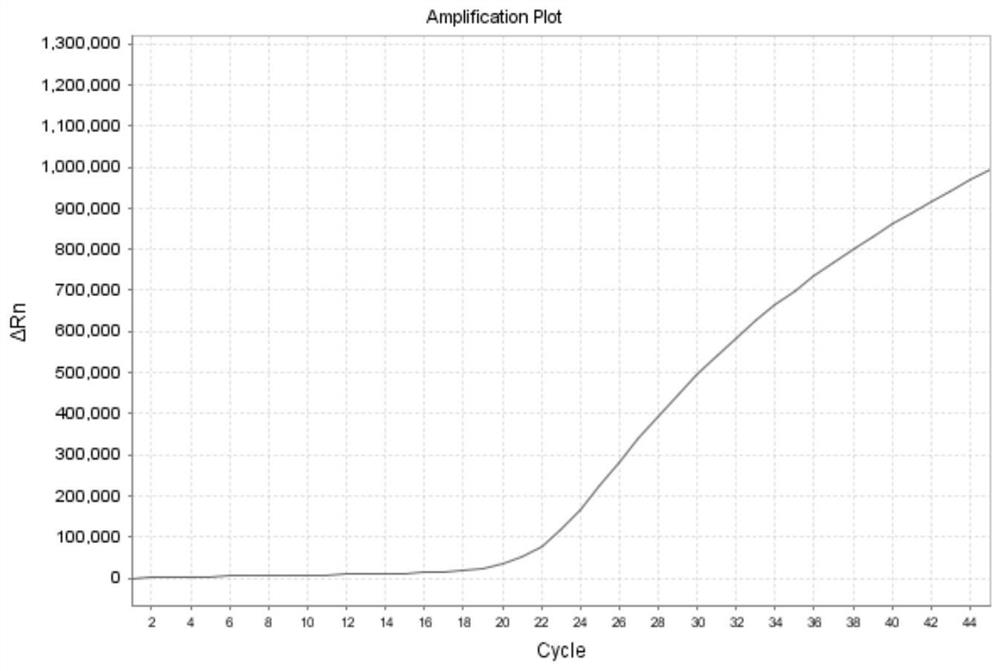
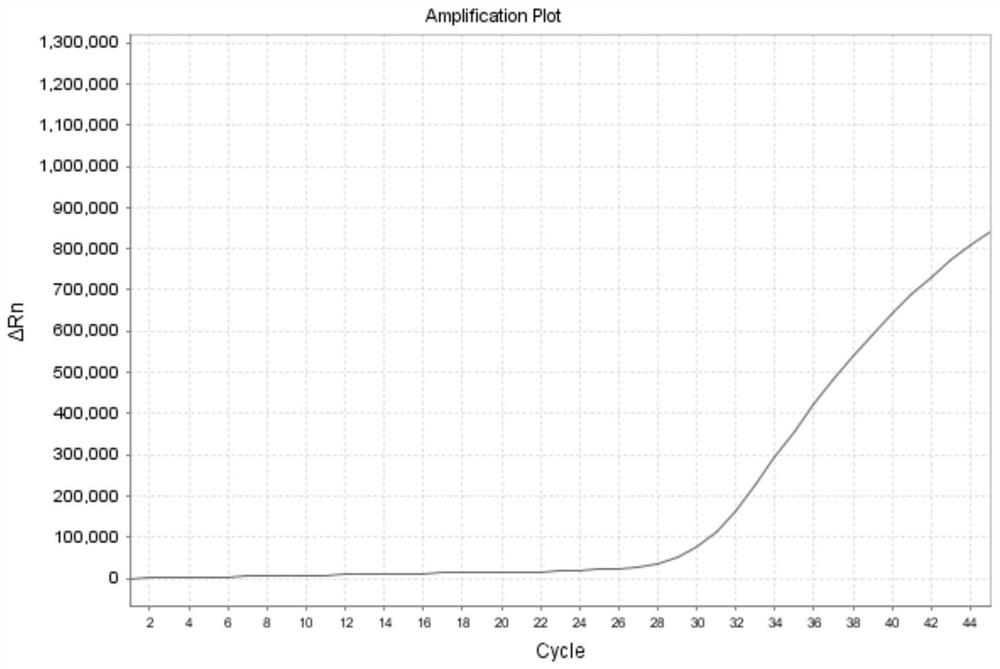
[0144]Example 2 adopts the same experimental method as Example 1, except that the probe-primer combinations used in the present invention are respectively S1 group, S2 group and S3 group, and the formulas of the probe-primer combination are respectively: (1) S1 group : The third primer set: upstream primer: 0.5 μl / test, downstream primer: 0.5 μl / test; fourth primer set: upstream primer: 0.5 μl / test, downstream primer: 0.5 μl / test, probe: 0.15 μl / test ; (2) S2 group: fifth primer group: upstream primer: 0 μl / test, downstream primer: 0.4 μl / test; sixth primer group: upstream primer: 0.9 μl / test, downstream primer: 0.4 μl / test, probe : 0.15μl / test; (3) S3 group: seventh primer group: upstream primer: 0μl / test, downstream primer: 0.4μl / test; eighth primer group: upstream primer: 0.9μl / test, downstream primer: 0.4μl / test, probe: 0.15 μl / test. The samples are influenza A (untyped) samples, and the results are as follows Figure 10 , Figure 11 , Figure 12 As shown, it shows th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com