Detection method of acetylcysteine enantiomer
A technology for acetylcysteine and enantiomers, applied in the field of analysis and detection, can solve the problems of low accuracy, insufficient separation of chromatographic peaks of isomers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
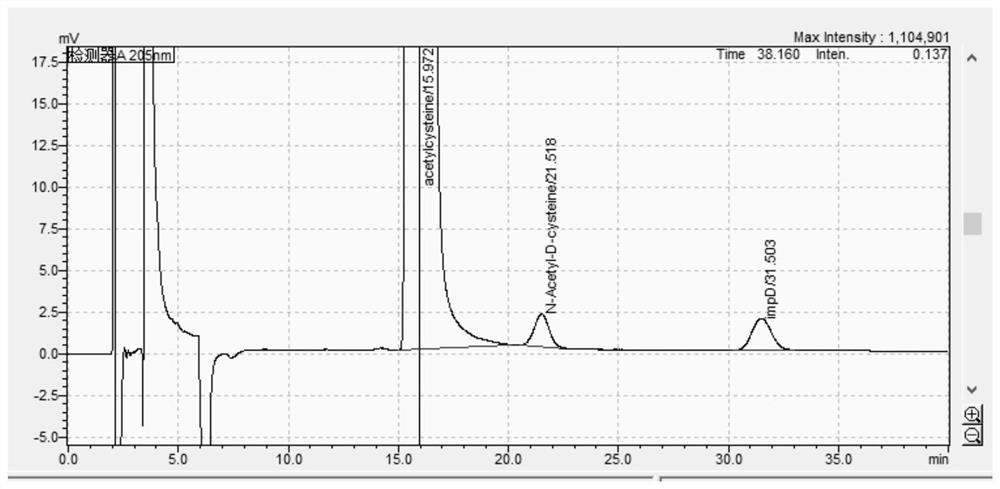
Image
Examples
Embodiment 1
[0037] 1. Prepare the solution
[0038] Test solution: Measure 1.5ml of Acetylcysteine Injection, put it in a 20ml measuring bottle, add diluent (ethanol-isopropanol=60:40v / v) to dilute to the mark, shake well, and use it as the test product solution.
[0039] Spiked test solution: Measure 1.5ml of Acetylcysteine Injection, put it in a 20ml volumetric bottle, add 2ml of the reference substance stock solution precisely, add diluent to dilute to the mark, shake well, and use it as the spiked test solution . 6 copies were prepared in parallel.
[0040] Reference substance solution: take 5.6 mg of the isomer reference substance, accurately weigh it, put it in a 25ml measuring bottle, add a diluent to dissolve and dilute to the mark, shake well, and use it as the reference substance stock solution, accurately measure 2ml of the reference substance stock solution, Put it in a 20ml measuring bottle, add diluent to dilute to the mark, shake well, and use it as a reference solut...
Embodiment 2
[0067] 1. Preparation of solution (same as Example 1)
[0068] 2. HPLC detection
[0069] Diluent: ethanol: isopropanol = 60:40 (volume ratio)
[0070] Mobile phase: n-hexane: isopropanol: ethanol: trifluoroacetic acid = 88:10:2:0.1 (volume ratio)
[0071] Column: CHIRALPAK IG, 4.6×250mm, 5μm
[0072] Detection wavelength: 205nm
[0073] Column temperature: 30℃
[0074] Flow rate: 1.5ml / min
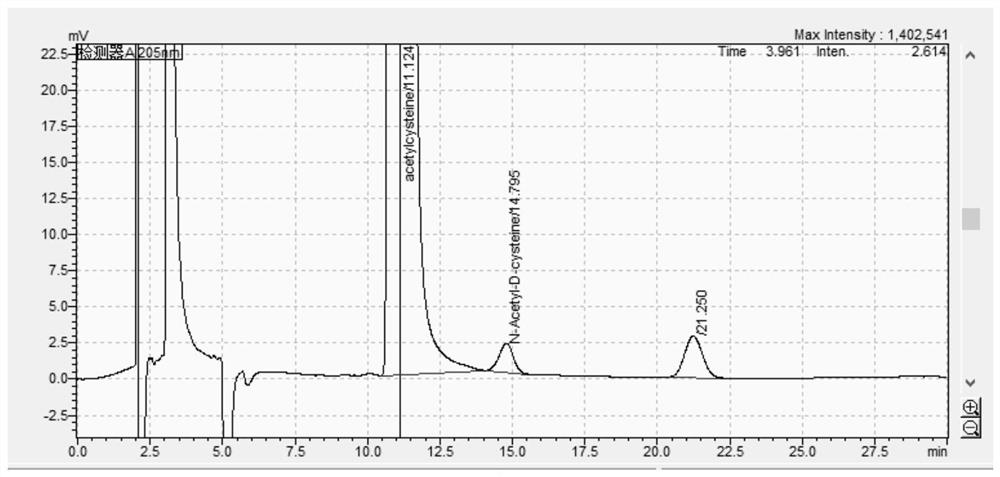
[0075] 2.1 Chromatogram
[0076] Precisely measure 10 μl of the test solution, inject the sample, and record the chromatogram as follows figure 2 . according to figure 2 , the number of plates is 5857, and the degree of separation is 3.8.
[0077] 2.2 Repeatability
[0078] Precisely measure 6 doses of 10 μL of the standard solution of the test sample, inject the sample, record the chromatogram, and calculate the result according to the chromatogram:
[0079] sample 1 2 3 4 5 6 RSD Recovery rate 100.8 101.9 103.2 101.0 102.8 102.5 0.96% degr...
Embodiment 3
[0087] 1. Preparation of solution (same as Example 1)
[0088] 2. HPLC detection
[0089] Diluent: ethanol: isopropanol = 60:40 (volume ratio)
[0090] Mobile phase: n-hexane: isopropanol: ethanol: trifluoroacetic acid = 92:6:2:0.1 (volume ratio)
[0091] Column: CHIRALPAK IG, 4.6×250mm, 5μm
[0092] Detection wavelength: 205nm
[0093] Column temperature: 30℃
[0094] Flow rate: 1.5ml / min
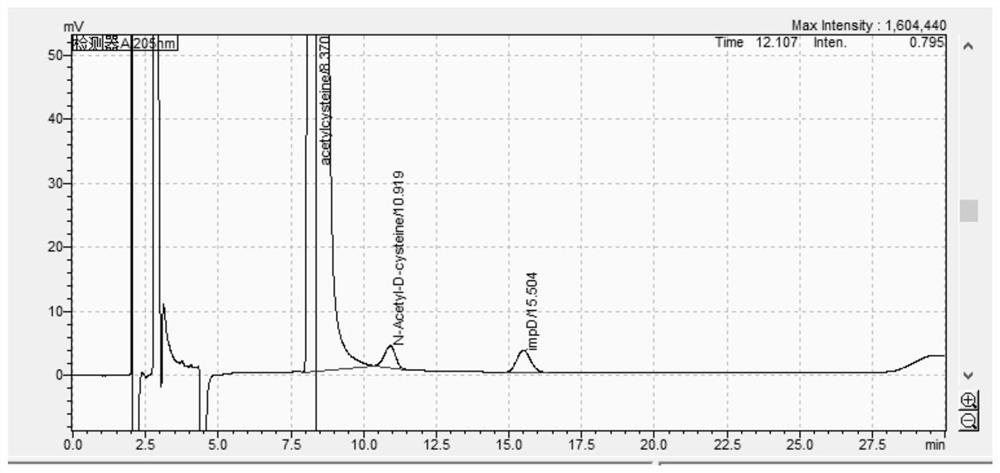
[0095] 2.1 Chromatogram
[0096] Precisely measure 10 μl of the test solution, inject the sample, and record the chromatogram as follows image 3 . according to image 3 , the number of trailing trays is 6633, and the degree of separation is 4.7.
[0097] 2.2 Repeatability
[0098] Precisely measure 6 doses of 10 μL of the standard solution of the test sample, inject the sample, record the chromatogram, and calculate the result according to the chromatogram:
[0099] sample 1 2 3 4 5 6 RSD Recovery rate 103.6 102.1 101.2 100.5 102.8 104.3 1.4% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com