Hypochlorous acid trigger activation type near-infrared fluorescent probe as well as preparation method and application thereof
A fluorescent probe, near-infrared technology, applied in the field of fluorescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Fc 2 -The synthetic route of CBDP is as follows:
[0062]
[0063] The specific process is as follows:
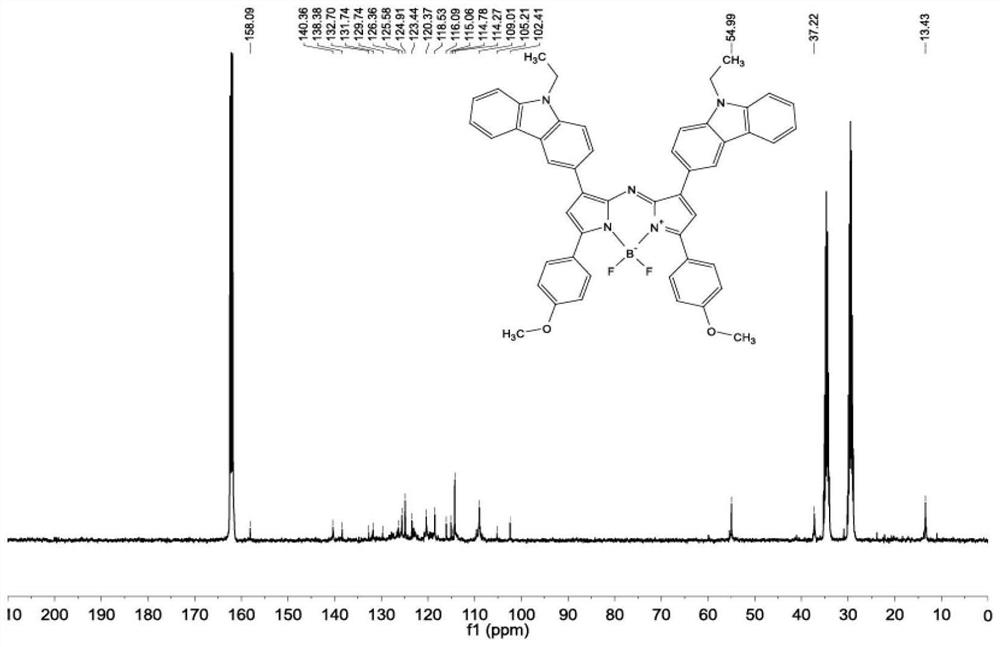
[0064] (1) Synthesis of compound A1: N-ethylcarbazole-3-carbaldehyde (3.55 g, 15.0 mmol), 3-acetyl-9-ethylcarbazole (3.30 g, 15.0 mmol), potassium hydroxide ( 2.52 g, 45 mmol) was dissolved in 100 mL of ethanol by ultrasonic, reacted at 60° C. overnight, then filtered with suction and washed twice with cold ethanol to obtain a yellow solid product A1 with a yield of 62%. 1 H NMR (400MHz, DMSO-d 6 ,δ,ppm)δ=9.18(s,1H),8.78(s,1H),8.40(d,J=7.8,1H),8.32(d,J=8.7,1H),8.28–8.19(m,2H ),8.07(d,J=8.6,1H),8.00(d,J=15.4,1H),7.76(d,J=8.7,1H),7.71(dd,J=8.2,6.2,2H),7.66( d, J=8.4, 1H), 7.57–7.46 (m, 2H), 7.30 (dt, J=14.9, 7.5, 2H), 4.50 (m, J=15.1, 7.5, 4H), 1.35 (m, J= 12.6, 6.9, 6H). 13 C NMR (101MHz, DMF-d 6 ,δ,ppm)δ=188.42,145.12,142.87,141.62,141.00,140.80, 130.14,127.65,127.17,127.14,126.98,126.71,125.11,123.40,122.97,122.92,122.76, 122.50,121.62,121.36,120.47,120.17...
Embodiment 2
[0068] Example 2: Fc 2 The synthetic route of -OBDP is as follows:
[0069]
[0070] The specific process is as follows:
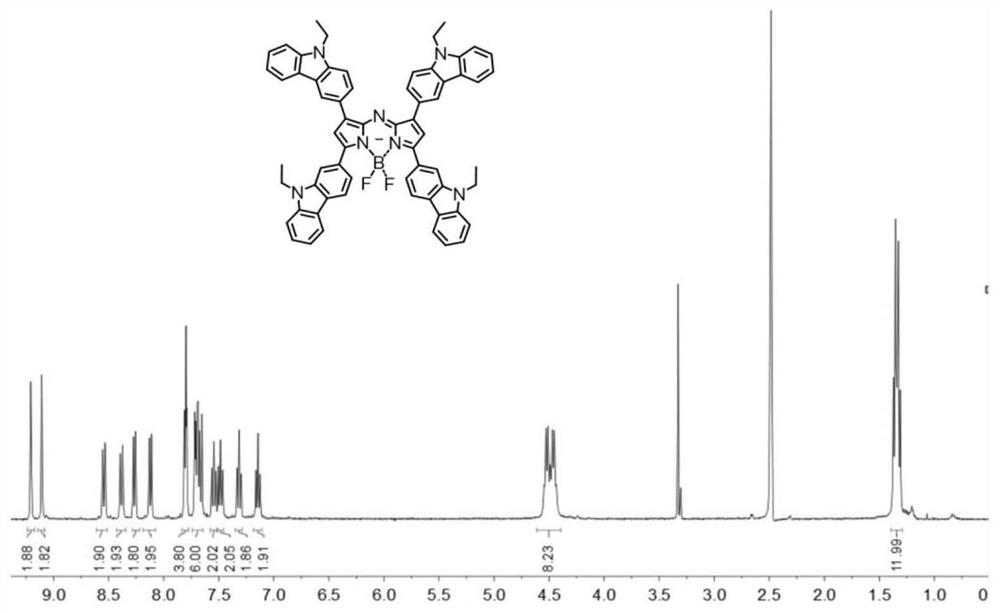
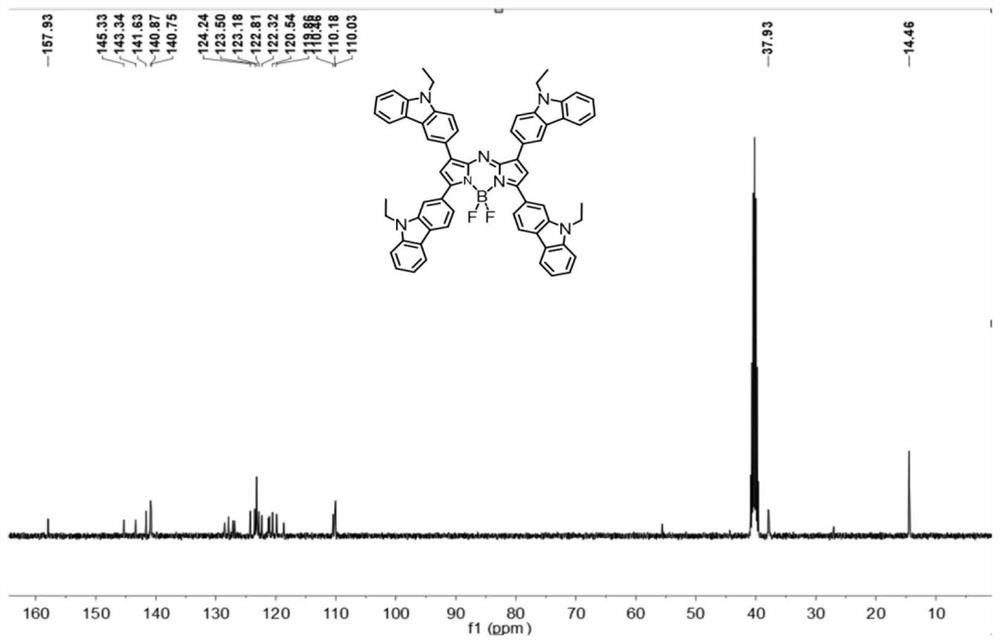
[0071] (1) Synthesis of compound B1: N-ethylcarbazole-3-carbaldehyde (2.2 g, 10.0 mmol), p-methoxyacetophenone (1.5 g, 10.0 mmol), potassium hydroxide (1.68 g, 30 mmol) ) was dissolved in 100 mL of ethanol by ultrasound, reacted overnight at 60° C., then suction filtered and washed twice with cold ethanol to obtain a yellow solid product B1 with a yield of 73%. 1 H NMR (400MHz, DMSO-d 6 ,δ,ppm)δ=8.73(s,1H),8.21(m,J=8.9,3H),7.97(dd,J=22.7,9.6,4H),7.68(d,J=8.6,1H),7.64 (d, J=8.2, 1H), 7.49 (t, J=8.1, 1H), 7.26 (t, J=7.4, 1H), 7.10 (d, J=8.9, 2H), 4.47 (q, J=7.0 , 2H), 3.87(s, 3H), 1.32(t, J=7.1, 3H). 13 C NMR (101MHz, DMSO-d 6 ,δ,ppm)δ=187.87,163.68,145.56,141.66,140.78,131.61, 131.42,127.63,126.97,126.49,123.37,122.96,122.57,121.31,120.17,119.35,114.64, 110.25,110.21,56.22,37.87,14.44 .MALDI-TOF-MS(m / z):calc.for[C 24 H 21 NO 2 Na] + , 378.4188...
Embodiment 3
[0076] First verify Fc 2 - Photostability of CBDP probes. like Figure 15 shown at 730nm laser (180mW / cm 2 ) irradiated for 3000s and found that the fluorescence intensity did not change significantly, while the fluorescence of ICG decreased significantly, indicating that Fc 2 -CBDP probe has good photostability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com