Tissue culture method of potentilla glandulifera
A technique of tissue culture and hair growth, which is applied in the field of plant tissue culture, can solve the problems of less raw materials required and large reproduction coefficient, and achieve the effects of good growth state, high culture efficiency, and convenient material collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Sterilization of explants
[0020] Taking the healthy plants of Cauliflower as the test material, cut young leaves, soak them in detergent water for 1 min, rinse them under running water for 30 min, put them on the ultra-clean workbench, disinfect them with 75% alcohol solution for 1 min, and then pour them out. After alcohol, rinse with sterile water for 3-4 times, then soak in 2% and 4% sodium hypochlorite solution for several minutes respectively (see Table 3.1 for the specific time), shake continuously during this period to make the explant and the solution fully contact, and then use Immerse in sterile water for 5-6 times, and dry the surface water with sterile filter paper. The leaves were cut into leaf discs of 0.5*0.5mm and inoculated on MS+6-BA0.5mg / L+2,4-D0.5mg / L medium. Each treatment was inoculated with 30 explants, repeated 3 times. After 3 weeks, the contamination rate, mortality rate and callus induction rate of different disinfection schemes w...
Embodiment 2
[0028] Example 2 Callus induction
[0029] The sterilized explants were inoculated into MS minimal medium supplemented with different concentrations of 6BA, 2,4D, NAA and TDZ, 30 explants per treatment in 3 replicates. After 30 days, the callus induction rate of the explants was counted, and the growth state and color of the callus were observed and recorded.
[0030] Table 3 Callus induction medium
[0031]
[0032]
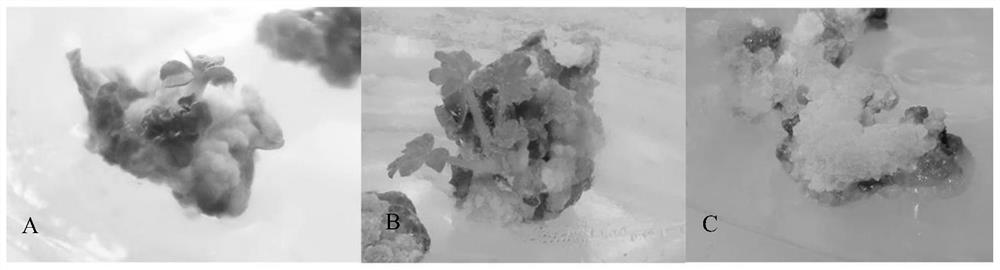
[0033] It can be seen from Table 4 that the ratios of hormones in the medium are different, and the callus induction rate and callus state of the corresponding leaves of C. chinensis are significantly different. The results showed that when a single cytokinin 6-BA was added to the medium, no callus could be produced on the leaves regardless of the concentration of 6-BA.
[0034]On each medium with different 6-BA and 2.4D mass concentrations, the callus induction rate of the leaves of C. pilaris was significantly different (P<0.05). The mass concentratio...
Embodiment 3
[0044] Example 3 Callus differentiation
[0045] The callus with vigorous growth was selected and transferred to the differentiation medium, and the medium ratio is shown in Table 5. 10 calli were inoculated for each treatment with 3 replicates. After 30 days, the callus differentiation rate was counted, and the growth state and color of the callus were observed and recorded. If there were small buds, the growth state of the buds was recorded.
[0046] Table 5 Callus Differentiation Medium
[0047]
[0048]
[0049] The callus in good condition induced by the leaves of C. pilaris was inoculated on the differentiation medium, and the statistical results after 30 days are shown in Table 3.6. According to the data in the table, it can be seen that different hormone ratios have the same effect on the different hormones. Vegetable callus differentiation has an important impact, only 4 of the 18 different mediums successfully differentiated callus into adventitious buds, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com