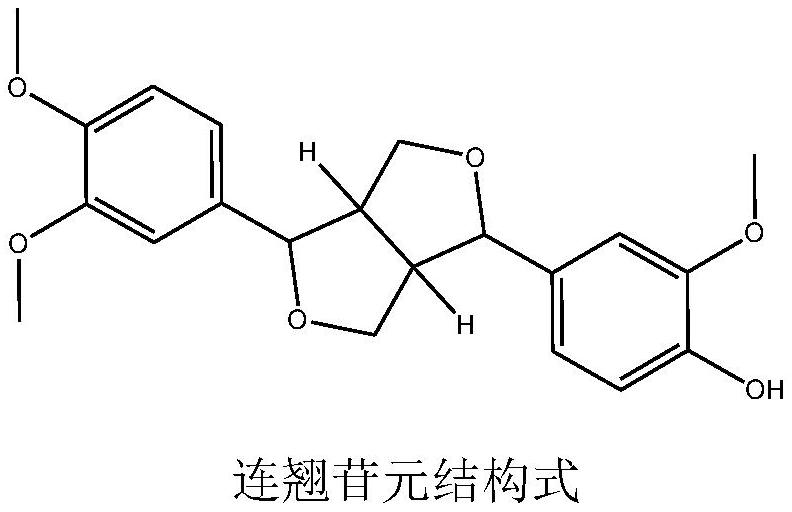
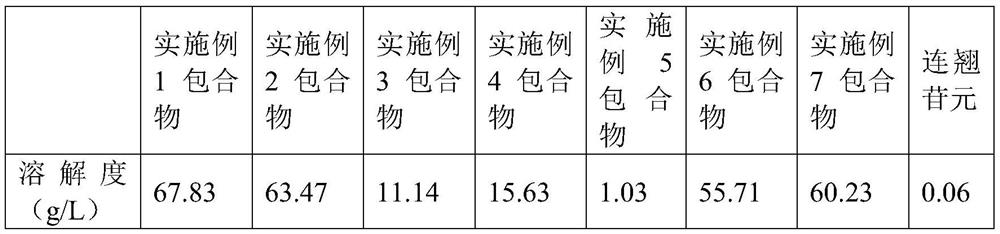
Inclusion compound of forsythin and cyclodextrin or cyclodextrin derivative and preparation method of inclusion compound
A technology of forsythia aglycone and cyclodextrin is applied in the directions of active ingredients of heterocyclic compounds, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of poor solubility of forsythia aglycone, increase biological Availability and other issues, to achieve the effects of high bioavailability, fast dissolution, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Forsythin: hydroxypropyl-β-cyclodextrin = 1:5.
[0023] Take 4 g of hydroxypropyl-β-cyclodextrin and put it in a beaker, add water to dissolve, put it on a magnetic heating stirrer, and stir at 60 °C, and add 0.8 g of forsythia aglycone. Filtration, freeze-drying of the filtrate to obtain loose powder, namely forsythia aglycone hydroxypropyl-β-cyclodextrin inclusion complex.
Embodiment 2
[0025] Forsythin: hydroxypropyl-β-cyclodextrin = 1:10.
[0026] Take 4 g of hydroxypropyl-β-cyclodextrin and put it in a beaker, add water to dissolve, to obtain solution A; take 2 g of forsythia aglycone, dissolve it with methanol under ultrasonic conditions, to obtain solution B; solution A is ultrasonicated at 40 °C, While adding solution B, after all the addition, continue to ultrasonic for 3h, filter, and dry the filtrate to obtain forsythia aglycone hydroxypropyl-β-cyclodextrin inclusion complex.
Embodiment 3
[0028] Forsythin: dimethyl-β-cyclodextrin = 1:10.
[0029] Take 40 g of dimethyl-β-cyclodextrin and put it in a beaker, add water to dissolve, to obtain solution A; take 4 g of forsythia aglycone, dissolve it with 60% ethanol solution under ultrasonic conditions, to obtain solution B; solution A is at 50 ℃ While sonicating, add solution B, after all the addition, continue sonicating for 1 h, filter, and spray-dry the filtrate to obtain forsythia aglycone β-dimethyl-β-cyclodextrin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com