Method for synthesizing chlorine trifluoride with high yield
A chlorine trifluoride, high-yield technology, applied in the field of high-yield synthesis of chlorine trifluoride, can solve the problems of complex process, low raw material purity and reaction conversion rate, low chlorine trifluoride yield, etc. Reasonably effective, excellent catalysis, improved purity and conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
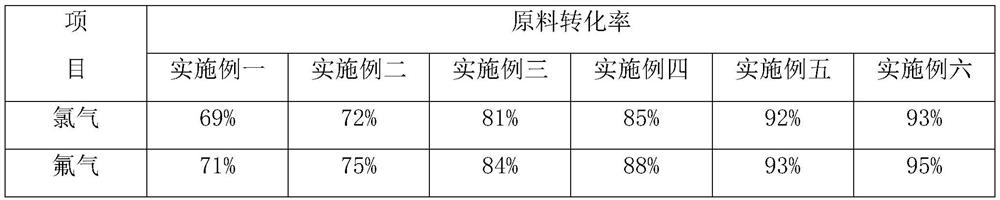
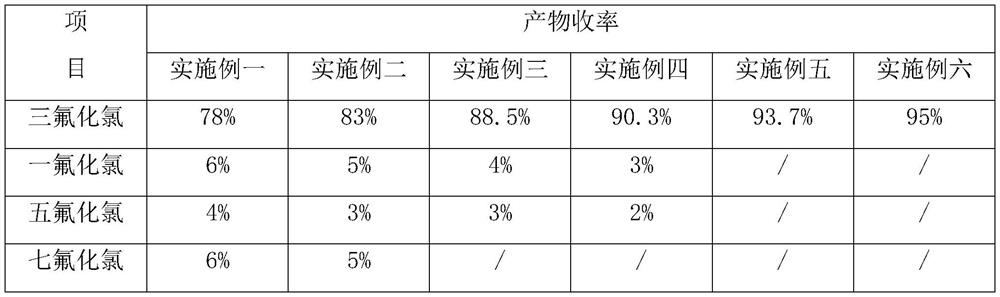
Image
Examples
Embodiment 1
[0045]A method for synthesizing chlorine trifluoride in high yield, the specific steps of the method are as follows:
[0046] Step 1: Set the pressure of the reaction system to 0.01-0.02Mpa, chlorine gas is metered into the dryer to be preheated to remove moisture at a temperature of 100-130°C, and fluorine gas is metered into the dryer to be preheated at a temperature of 100-130°C to remove moisture. removal of hydrogen fluoride;
[0047] Step 2: The inert gas argon is introduced into the reactor to be F 2 Carrier gas, the reactor is charged with anhydrous NiF as a catalyst for synthesizing chlorine trifluoride 2 ;
[0048] Step 3: Control the temperature of chlorine gas and fluorine gas at 100-130 °C, and press chlorine gas and fluorine gas by Cl 2 / F 2 The ratio of = 1 / 1 is passed into the gas mixing chamber in the reactor, and the gas flow rate is reduced to 0.01-0.02m / s for buffer mixing to control the F in the mixed gas. 2 The concentration range of 20-40%;
[0049...
Embodiment 2
[0057] A method for synthesizing chlorine trifluoride in high yield, the specific steps of the method are as follows:
[0058] Step 1: Set the pressure of the reaction system to 0.01-0.02Mpa, chlorine gas is metered into the dryer to be preheated to remove moisture at a temperature of 100-130°C, and fluorine gas is metered into the dryer to be preheated at a temperature of 100-130°C to remove moisture. removal of hydrogen fluoride;
[0059] Step 2: The inert gas nitrogen introduced into the reactor is F 2 Carrier gas, the reactor is charged with anhydrous NiF as a catalyst for synthesizing chlorine trifluoride 2 ;
[0060] Step 3: Control the temperature of chlorine gas and fluorine gas at 100-130 °C, and press chlorine gas and fluorine gas by Cl 2 / F 2 The ratio of =1 / 2.5 is passed into the gas mixing chamber in the reactor, and the gas flow rate is reduced to 0.01-0.02m / s for buffer mixing, and the F in the mixed gas is controlled. 2 The concentration range of 20-40%; ...
Embodiment 3
[0069] A method for synthesizing chlorine trifluoride in high yield, the specific steps of the method are as follows:
[0070] Step 1: Set the pressure of the reaction system to 0.1~0.2Mpa, chlorine gas is metered into the dryer to be preheated to remove moisture at a temperature of 100~130℃, and fluorine gas is metered into the dryer to be preheated and removed at a temperature of 100~130℃. removal of hydrogen fluoride;
[0071] Step 2: The inert gas argon is introduced into the reactor to be F 2 Carrier gas, the reactor is charged with anhydrous NiF as a catalyst for synthesizing chlorine trifluoride 2 ;
[0072] Step 3: Control the temperature of chlorine gas and fluorine gas at 100-130 °C, and press chlorine gas and fluorine gas by Cl 2 / F 2 The ratio of = 1 / 3.2 is passed into the gas mixing chamber in the reactor, and the gas flow rate is reduced to 0.05-0.1m / s buffer mixing to control the F in the mixed gas. 2 The concentration range of 20-40%;
[0073] Step 4: Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com