Bispecific antibody and application thereof in cancer treatment
A bispecific antibody and a specific technology, applied in the field of bispecific antibody and its application in the treatment of cancer, can solve problems such as B cell depletion, and achieve the effects of reducing the secretion of immune factors, reducing toxic side effects, and prolonging the half-life in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of CD3 single domain antibody
[0032] Select healthy adult alpacas, mix the recombinant human CD3 antigen (preserved in our laboratory) and Freund's complete adjuvant at a ratio of 1:1, and subcutaneously inject the alpacas on the back for four times in total. The immunization interval is Two weeks. After successful immunization, 10 mL of alpaca peripheral blood was collected to construct a phage display library.
[0033] Lymphocytes were separated from the collected peripheral blood of alpaca using a lymphocyte separation kit (Sigma-Aldrich); 1 × 10 7 The total RNA was extracted by the Trizol method. The steps were as follows: add 1 mL of Trizol (purchased from Sigma) to the EP tube containing lymphocytes, pipet repeatedly, and place on ice for 5 minutes; add 250 μL of chloroform, vortex for 30 seconds, and continue Place on ice for 5 minutes; centrifuge at 12,000g for 15 minutes at 4°C, transfer the aqueous phase to a new EP tube; add an equal...
Embodiment 2
[0042] Example 2 Design and preparation of bispecific antibodies
[0043] 2.1 Design of bispecific antibodies
[0044] The traditional structural antibodies targeting CD19 and CD3 were preserved by our laboratory, and their nucleic acid sequences were cloned into the PTT5 vector, wherein the antibody targeting CD19 had HCDR1 as shown in SEQ ID NO: 1, and as shown in SEQ ID NO: 2. HCDR2 shown in SEQ ID NO:3, LCDR1 shown in SEQ ID NO:4, LCDR2 shown in SEQ ID NO:5, and LCDR3 shown in SEQ ID NO:6; and Has a heavy chain variable region shown in SEQ ID NO:10 and a light chain variable region shown in SEQ ID NO:11.
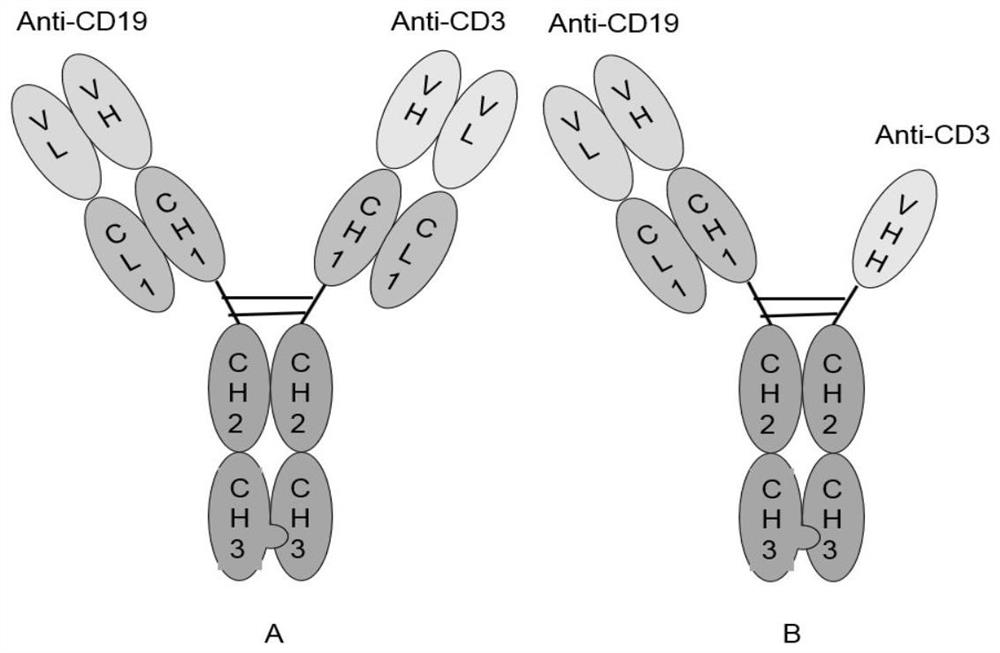
[0045] In this example, traditional CD3-targeting antibodies and single-domain antibodies were used to construct bispecific antibodies, so as to verify the corresponding anti-tumor effects. like figure 1 As shown in A, CD19-CD3 VH / VL BsAbs have traditional antibody structures and bind to CD19 and CD3 targets, respectively; as shown in figure 1 As shown in B, the CD19...
Embodiment 3
[0048] Example 3 In vitro anti-tumor experiments of bispecific antibodies
[0049] 3.1 Benefit cell isolation and culture
[0050] Human PBMC cells were isolated by Ficoll density gradient centrifugation: 10 mL of fresh human peripheral blood was drawn, mixed with 10 mL of serum-free RPMI1640 medium, and slowly added to the upper layer of 10 mL of density gradient centrifugation fluid Ficoll; centrifuged at 12,000 rpm for 10 min at room temperature; taken out the centrifuge tube, discarded Remove the upper layer of plasma, and then carefully aspirate the white layer PBMC between the plasma and Ficoll, and place it in a 50 mL centrifuge tube; add 15 mL of serum-free RPMI1640 medium, resuspend and centrifuge at 3000 rpm for 10 min, repeat the operation twice; add 15 mL of 10% serum-containing RPMI1640 medium at 37°C 5% CO 2 cultured under conditions.
[0051] 3.2 Tumor cell culture
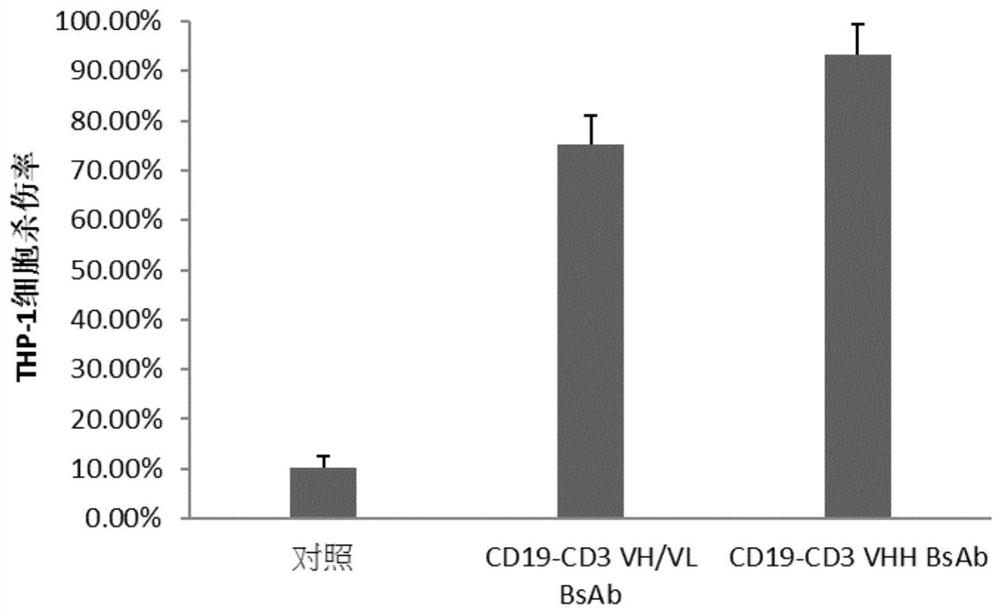
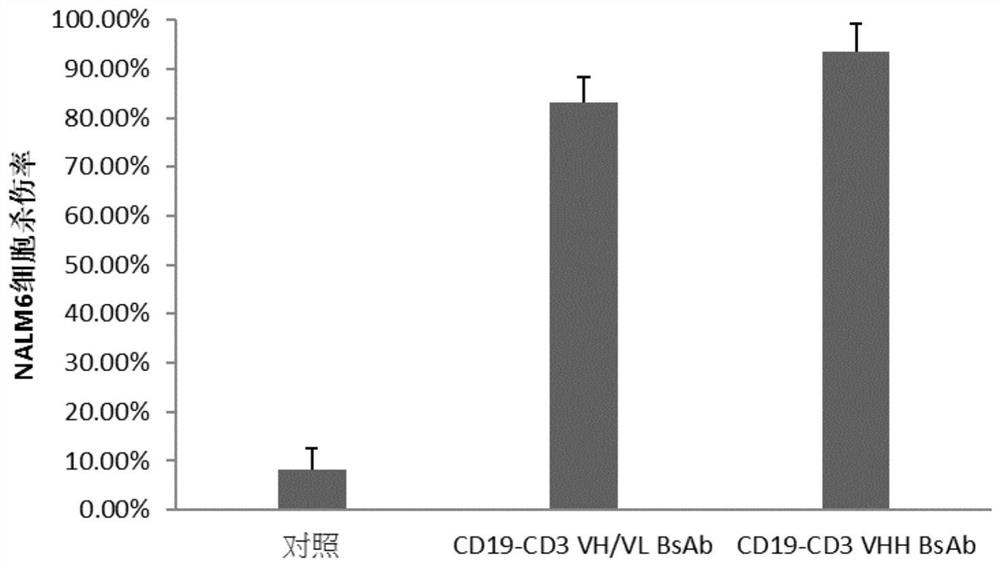
[0052] In the present invention, human acute myeloid leukemia cell line THP-1, acute lymphocy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com