Boron-modified carbon nitride material as well as preparation method and application thereof
A carbon nitride, atomic technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of easy deactivation, low reactivity, poor product selectivity, etc., and achieves a simple preparation method, The effect of convenient synthesis method, strong adsorption and ability to activate O2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
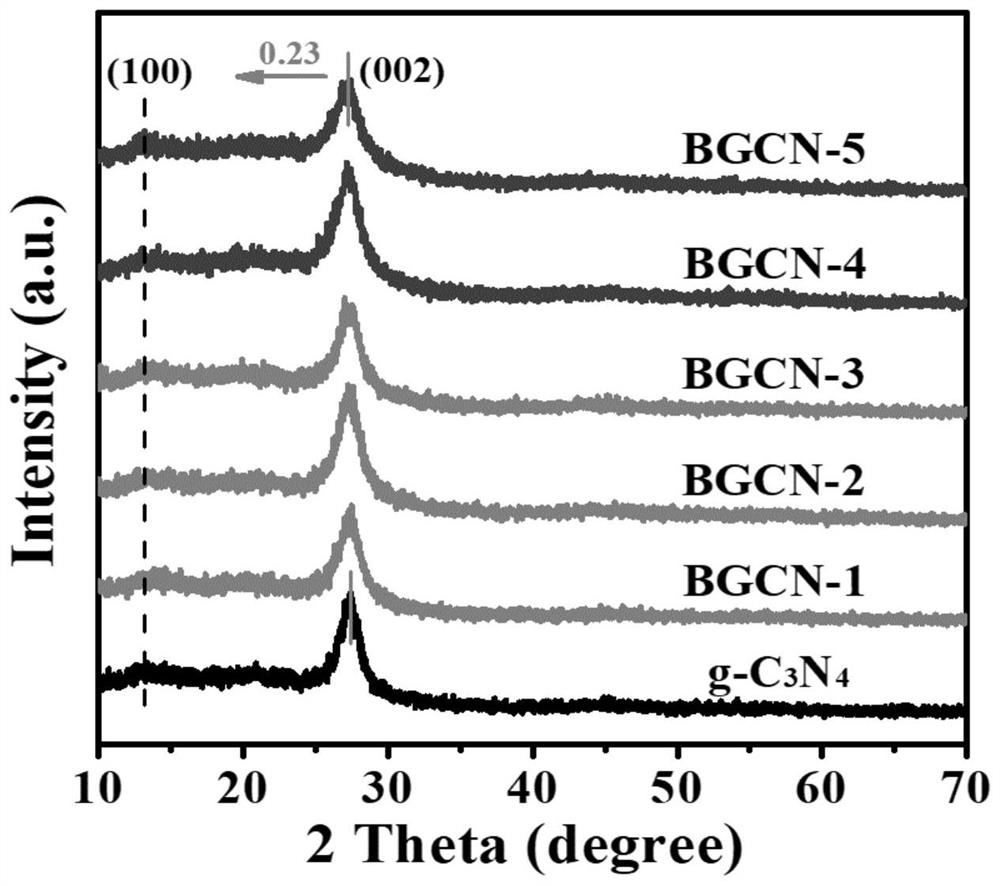
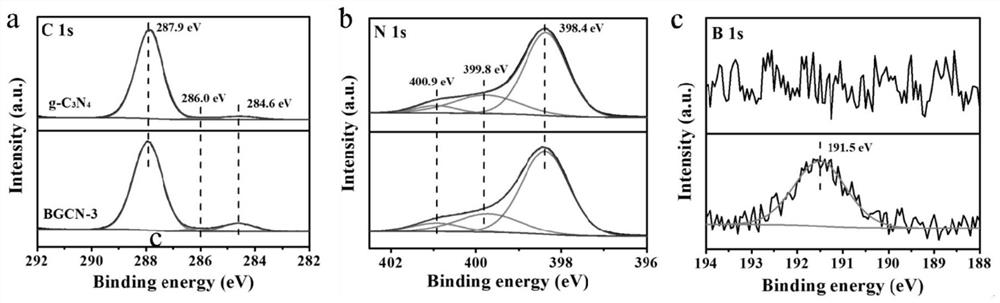
[0028] In a second aspect, the present invention provides a method for preparing the boron-modified carbon nitride material, which includes the steps of: mixing urea and boric acid solution, evaporating the solvent, recrystallization, and calcining the recrystallized mixture, so that B atoms are replaced by sp in the 3-s-triazine ring 2 Hybridized C atoms into g-C 3 N 4 In the framework, boron-modified carbon nitride materials were prepared.
[0029] The B atom is replaced by sp in the 3-s-triazine ring 2 Hybridized C atoms into g-C 3 N 4 in the skeleton and does not affect g-C 3 N 4 Basic crystal structure. g-C obtained by mixing calcined urea and boric acid 3 N 4 Compared with g-C obtained by simply calcining urea 3 N 4 It has more excellent visible light responsiveness and carrier separation efficiency, and can significantly inhibit the recombination of photogenerated electron-hole pairs.
[0030] In some embodiments, the solvent is water or ethanol.
[0031] In ...
Embodiment 1
[0045] A boron-modified g-C 3 N 4 Photocatalyst and preparation method thereof, comprising the following steps:
[0046] (1) 10g of urea and 10mg of boric acid were dissolved in 30mL of deionized water, and vigorously stirred for 30min;
[0047] (2) Transfer the above mixed solution to a heating table and heat to evaporate the solvent, recrystallize, and evaporate at a temperature of 80°C for 12h;
[0048] (3) Grind the obtained recrystallization mixture uniformly, and heat it at 550°C for 5°C min -1 The heating rate was calcined in Air atmosphere for 4h;
[0049] (4) After the muffle furnace was naturally cooled, the obtained sample was washed with a large amount of deionized water to remove residual boric acid and incompletely polymerized soluble substances, and dried in an oven at 60°C.
Embodiment 2
[0051] The difference from Example 1 is that 1 mg of boric acid is added in step (1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com