Nickel-lanthanum oxide catalyst for methane dry reforming and preparation method thereof
A methane dry reforming and catalyst technology, which is applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., and can solve the problems of carbon deposition and high temperature sintering of active components. , to achieve high catalytic stability, increase specific surface area, and solve the effects of high temperature sintering and carbon deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
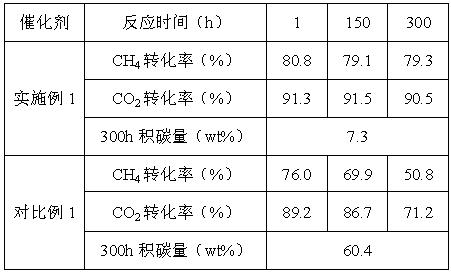
Examples
Embodiment 1
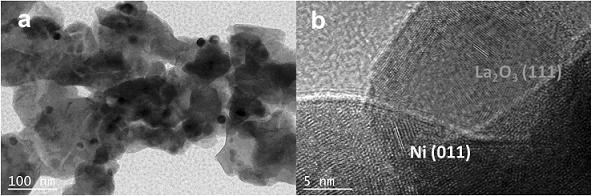
[0032] Carrier preparation: In a 1 L beaker, 13 g La (NO 3 ) 3 ·6H 2 O was dissolved in 200 mL of water with stirring, and then 1.0 mol·L was added dropwise. -1 NH 4 HCO 3 The precipitant adjusts the pH of the mixed slurry to 8.0. After continuing to stir for 0.5 h, the reactant was filtered and washed until the pH of the filtrate was 7.0, dried in an oven at 120 °C overnight, and finally calcined in a muffle furnace at 600 °C for 2.0 h to obtain the catalyst carrier La 2 O 2 CO 3 ;
[0033] Catalyst preparation: 3.0 g of the above La was added to a 50 mL crucible under infrared light irradiation 2 O 2 CO 3 carrier, weigh 0.30g Ni (NO 3 ) 2 ·6H 2 O was dissolved in 7 mL of water to obtain a precursor solution. The precursor solution was added to the crucible and immersed while stirring. After the solvent was evaporated to dryness, it was dried in an oven at 120 °C overnight, and calcined in a muffle furnace at 750 °C for 2.0 h. in tube furnace H 2 / N 2 (volume...
Embodiment 2
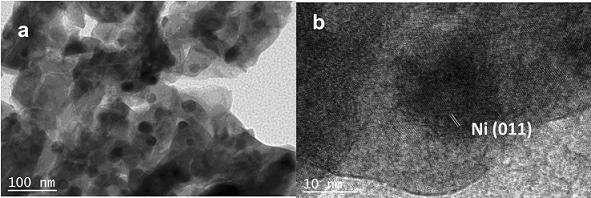
[0035] Carrier preparation: In a 1 L beaker, at room temperature (around 25 °C), 24 g La (CH 3 COO) 3 ·5H 2 O was dissolved in 200 mL of water with stirring, and then 0.5 mol L was added dropwise. -1 NH 3 ·H 2 O precipitant adjusts the pH of the mixed slurry to 8.0. After stirring for 0.5 h, the reactant was filtered and washed until the pH of the filtrate was 7.0, dried in an oven at 120 °C overnight, and finally calcined in a muffle furnace at 600 °C for 3.0 h to obtain the catalyst carrier La 2 O 2 CO 3 ;
[0036] Catalyst preparation: 3.0 g of the above La was added to a 50 mL crucible under infrared light irradiation 2 O 2 CO 3 carrier, weigh 0.40g Ni (CH 3 COO) 2 ·4H 2 O was dissolved in 7 mL of water to obtain a precursor solution. The precursor solution was added to the crucible and immersed while stirring. After the solvent was evaporated to dryness, it was dried in an oven at 120 °C overnight, and calcined in a muffle furnace at 800 °C for 2.0 h. in tu...
Embodiment 3
[0038] Carrier preparation: In a 1 L beaker, add 10 g LaCl at room temperature (around 25 °C) 3 Stir to dissolve in 200 mL of water, and then dropwise add 2.0 mol L -1 (NH 4 ) 2 CO 3 The precipitant adjusts the pH of the mixed slurry to 9.0. After continuing to stir for 0.5 h, the reactant was filtered and washed until the pH of the filtrate was 7.0, dried in an oven at 120 °C overnight, and finally calcined in a muffle furnace at 600 °C for 4.0 h to obtain the catalyst carrier La 2 O 2 CO 3 ;
[0039] Catalyst preparation: 3.0 g of the above La was added to a 50 mL crucible under infrared light irradiation 2 O 2 CO 3 Carrier, weigh 0.43g NiCl 2 ·6H 2 O was dissolved in 7 mL of water to obtain a precursor solution. The precursor solution was added to the crucible and immersed while stirring. After the solvent was evaporated to dryness, it was dried in an oven at 120 °C overnight, and calcined in a muffle furnace at 600 °C for 2.0 h. in tube furnace H 2 / N 2 (vol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com