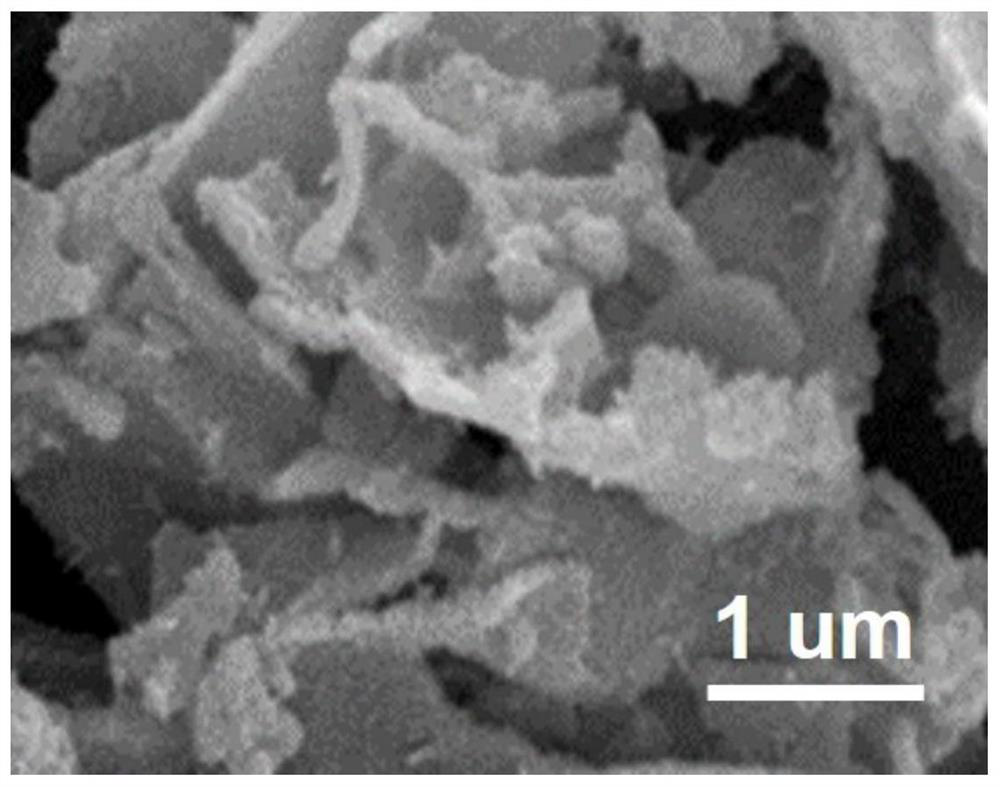
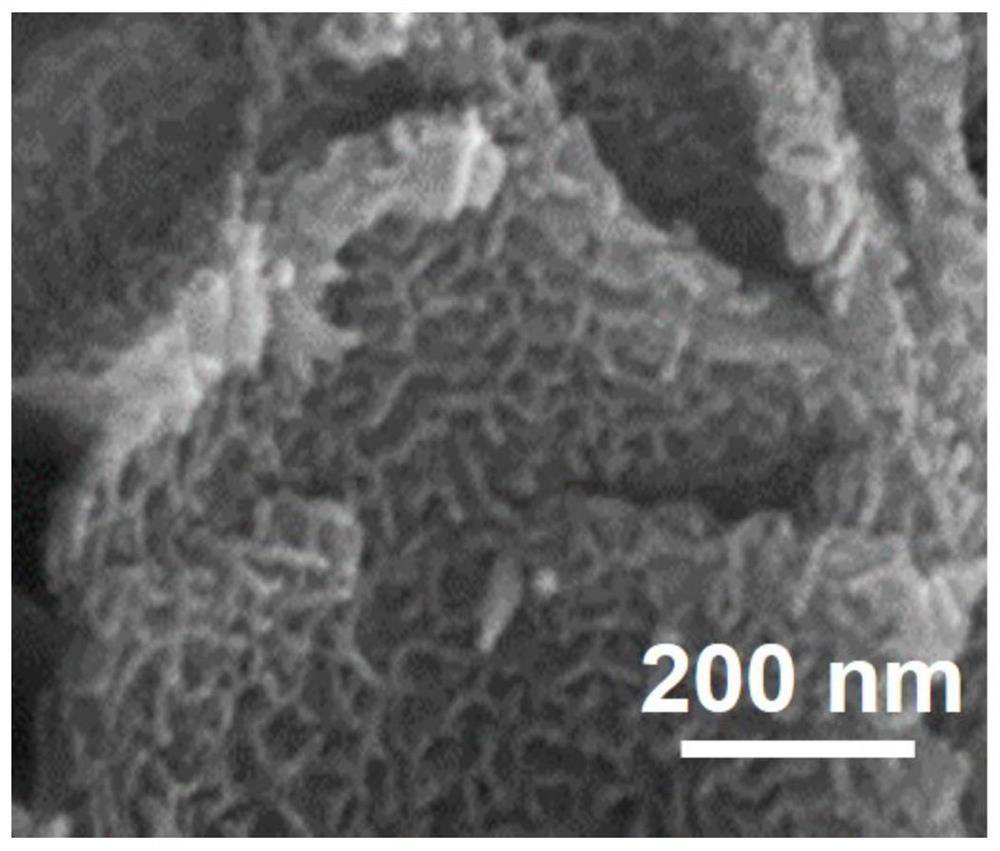
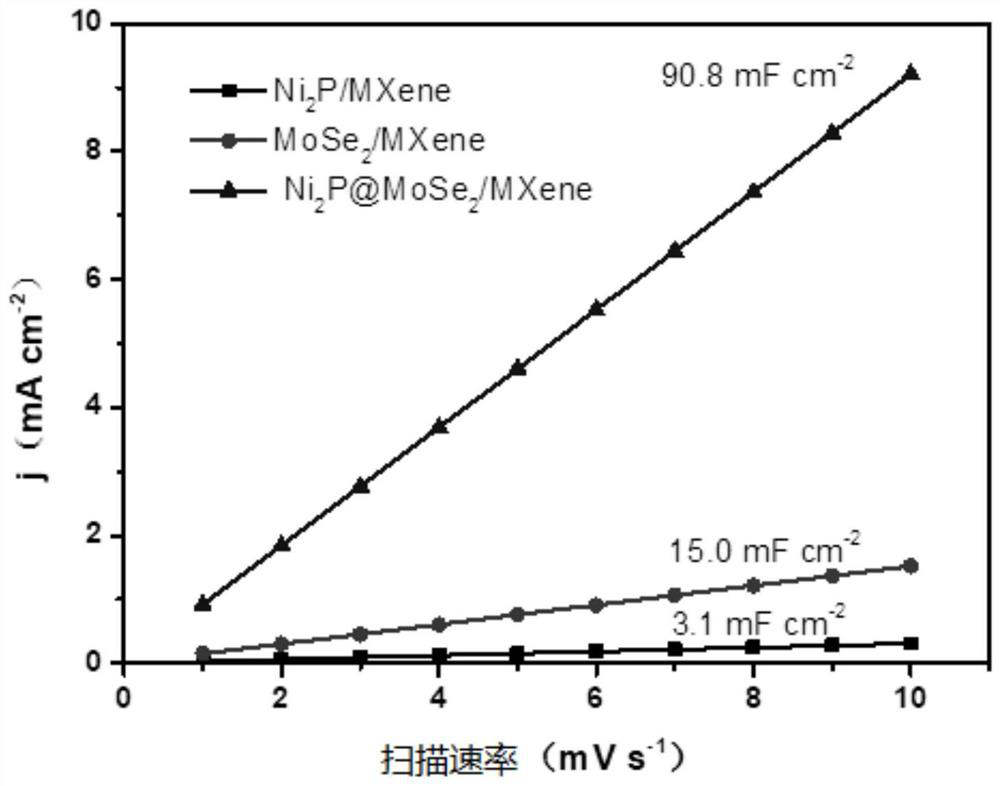
Heterogeneous catalyst, preparation method thereof and application of heterogeneous catalyst in hydrogen evolution by electrolyzing water
A heterogeneous catalyst, catalyst technology, applied in electrolysis components, electrolysis process, nanotechnology for materials and surface science, etc., can solve the problems of low cost performance and high price of precious metal catalysts, achieve performance improvement, excellent electrocatalytic performance, The effect of improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0070] Preparation Example 1 Carrier MXene (Ti 3 C 2 ) preparation
[0071] By etching Ti 3 AlC 2 Powder synthesis of MXene. First, 1 g of LiF was dissolved in 20 ml of HCl (9 mol / L), and then the solution was thoroughly mixed to obtain an etching solution. Next, 1 g of Ti 3 AlC 2 Slowly added to the etching solution and magnetically stirred at 35°C for 24h to obtain a solid. The resulting solid residue was then washed with deionized water and centrifuged at 3500 rpm until pH reached 6. The resulting precipitated product was dispersed in 150 ml of deionized water and then sonicated for 1 h in flowing argon. A few-layer MXene dispersion was obtained by collecting the supernatant after centrifugation at 3500 rpm for 1 hour.
Embodiment 1
[0073] (1) Ni 2 Preparation of precursors of P / MXene:
[0074] Ni was prepared by hydrothermal method 2 Precursor of P / MXene. 0.5816g Ni (NO 3 ) 2 ·6H 2 O (2 mmol), 0.6 g urea (10 mmol) and 0.222 g NH 4 F (6 mmol) was dissolved in 30 mL of deionized water and stirred for 20 min to form a homogeneous solution. Then, the MXene dispersion liquid obtained in Preparation Example 1 was placed in the above solution, and oxygen was excluded with argon gas, and transferred to a stainless steel autoclave together, and placed in an oven at 120° C. for 6 hours. After cooling to room temperature, washing with deionized water, and then drying in an oven at 60 °C for 6 h, the precursor I was obtained. Precursor I and 2.0 g NaH 2 PO 2 Put them on two porcelain boats, respectively placed downstream and upstream of the tube furnace, heated to 300 °C at a rate of 5 °C / min, and kept for 2 h for phosphating treatment. Finally, Ni was obtained after cooling to room temperature 2 P / MXene ...
Embodiment 2
[0078] (1) Ni 2 Preparation of precursors of P / MXene:
[0079] Ni was prepared by hydrothermal method 2 Precursor of P / MXene. 0.5816g Ni (NO 3 ) 2 ·6H 2 O (2 mmol), 0.6 g urea (10 mmol) and 0.222 g NH 4 F (6 mmol) was dissolved in 30 mL of deionized water and stirred for 20 min to form a homogeneous solution. Then, the MXene dispersion liquid obtained in Preparation Example 1 was placed in the above solution, and oxygen was excluded with argon gas, and transferred to a stainless steel autoclave together, and placed in an oven at 120° C. for 6 hours. After cooling to room temperature, washing with deionized water, and then drying in an oven at 60 °C for 6 h, the precursor I was obtained. Precursor I and 2.5 g NaH 2 PO 2 Put them on two porcelain boats, respectively placed downstream and upstream of the tube furnace, heated to 350 °C at a rate of 5 °C / min, and kept for 2 hours for phosphating treatment. Finally, Ni was obtained after cooling to room temperature 2 P / MX...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com