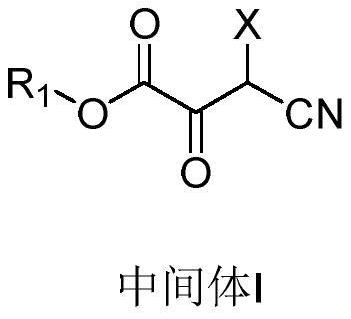
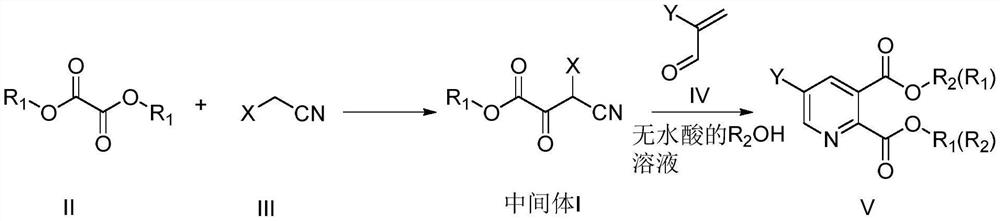
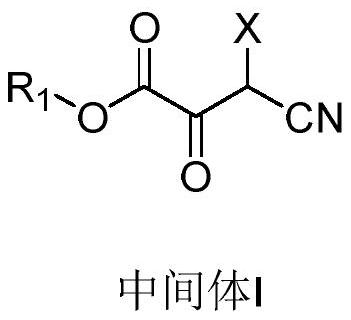
Preparation method of 2, 3-dipicolinic acid ester derivative intermediate and 2, 3-dipicolinic acid ester derivative
A technology of dicarboxylic acid esters and derivatives, which is applied in the field of pesticide chemistry, can solve the problems of many side reactions, unfavorable industrial production, and difficult to control reactions, and achieves the effect of high atom utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] ① Preparation of methyl 3-chloro-3-cyano-2-oxopropionate:
[0036]
[0037] 118.09 g (1 mol) of dimethyl oxalate and 180.1 g of 30% sodium methoxide methanol solution (1 mol) were stirred and dissolved at room temperature, and 90.60 g of chloroacetonitrile (1.2 mol) was slowly added dropwise at 15 to 20°C for 3 hours. , and stirring for 1 h after the addition was completed. After sampling qualified, it was cooled to 5~10 ℃, slowly added 182.5g of 30% hydrochloric acid methanol solution (1.5mol), acidified to pH value less than 4, filtered, evaporated to dryness methanol to obtain 160g of 3-chloro-3-cyano-2 -Methyl oxopropionate, qualitative content 94%, yield 93.4%. 1 H NMR(400MHz,DMSO)δ7.07(s,1H),3.83(s,3H).
[0038] ② Preparation of methyl 5-methylpyridine-2,3-dicarboxylate:
[0039]
[0040] 160g of 94% methyl 3-chloro-3-cyano-2-oxopropionate (0.93mol) obtained in step (1) was added to 136g of 30% hydrochloric acid methanol solution (1.1mol), and the tempera...
Embodiment 2
[0042] ① Preparation of methyl 3-chloro-3-cyano-2-oxopropionate:
[0043]
[0044] 118.09 g of dimethyl oxalate (1 mol) and 270.1 g of 30% sodium methoxide methanol solution (1.5 mol) were stirred and dissolved at room temperature, and 98.15 g of chloroacetonitrile (1.3 mol) was slowly added dropwise at 15 to 20°C. 3h, keep stirring for 1h after the dropwise addition. After sampling qualified, the temperature was lowered to 5-10° C., 304.17g of 30% hydrochloric acid methanol solution (2.5mol) was slowly added, and the pH value was acidified to less than 4 to obtain 593.89g of 3-chloro-3-cyano-2-oxopropane The acidification reaction solution of methyl acid is directly carried out to the next step without separation.
[0045] ② Preparation of methyl 5-methylpyridine-2,3-dicarboxylate:
[0046]
[0047]The acidification reaction solution of 593.89g of methyl 3-chloro-3-cyano-2-oxopropionate obtained in step 1 in Example 2 was heated to 60°C, and 78.2g of 2-methacrolein ( ...
Embodiment 3
[0049] ① Preparation of ethyl 3-methoxy-3-cyano-2-oxopropionate:
[0050]
[0051] Dissolve 146.14 g of diethyl oxalate (1 mol) and 102.1 g of sodium ethoxide (1.5 mol) in 500 g of toluene, and slowly add 78.1 g of chloroacetonitrile (1.03 mol) dropwise at 30 to 35°C for 2 hours. After the dropwise addition was completed, the mixture was kept stirring for 2h. After sampling qualified, it was cooled to 5-10°C, slowly added 158.28g of 30% hydrochloric acid ethanol solution (1.3mol), acidified to a pH value of less than 4, filtered, and evaporated to dryness to obtain 154.04g of 3-methoxy-3-cyanide Ethyl-2-oxopropionate, qualitative content 95%, yield 85.5%. 1 H NMR (400MHz, DMSO) δ 10.85(s, 1H), 4.28-4.22(m, 2H), 3.71(s, 3H), 1.26(t, J=7.1Hz, 3H).
[0052] ② Preparation of ethyl 5-ethylpyridine-2,3-dicarboxylate:
[0053]
[0054] 154.04g of 95% ethyl 3-methoxy-3-cyano-2-oxopropionate obtained in step ① of Example 3 was added to 117.5g of 30% hydrochloric acid ethanol s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com