Intravascular stent system and preparation method thereof
A vascular stent and transportation system technology, applied in the field of vascular stent system and its preparation, can solve the problems of vascular restenosis, vascular wall damage, etc., and achieve the effect of reducing the feeling of intervention, low delivery resistance, and good support strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
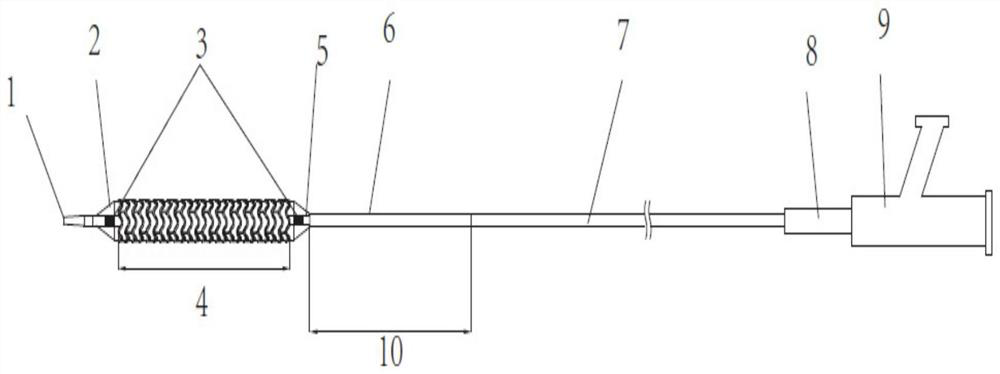
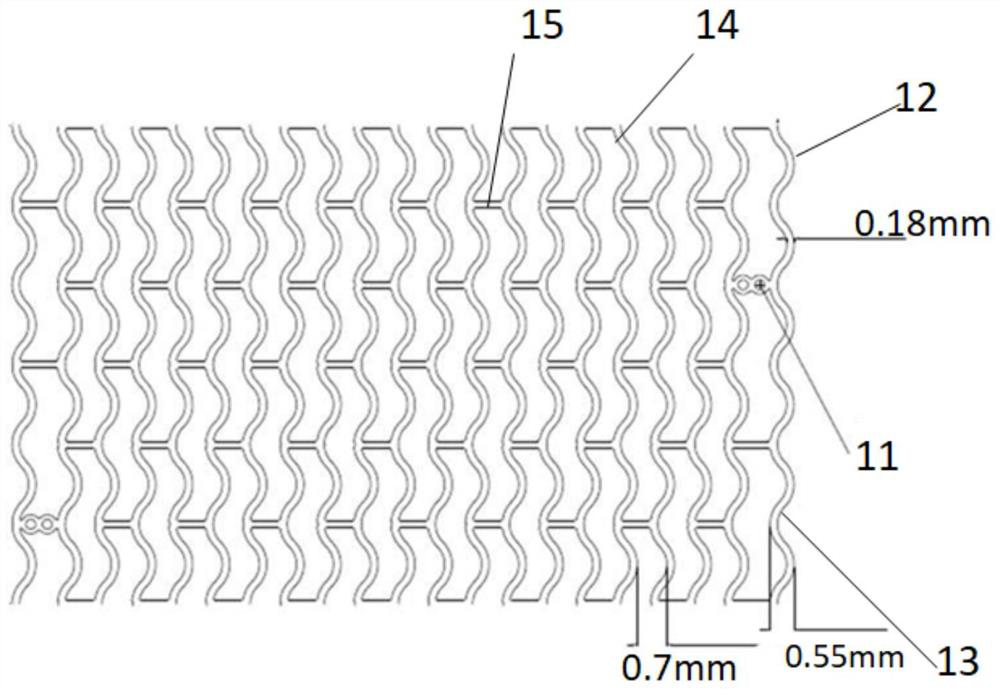
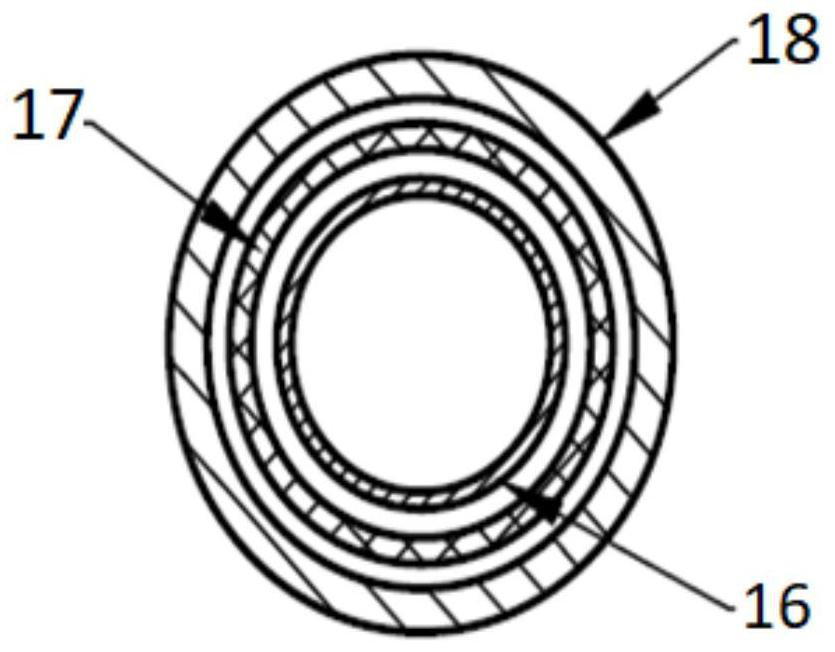
[0059] like figure 1 As shown, a vascular stent system includes a stent and a balloon delivery system for transporting the stent to the lesion. The balloon delivery system includes a balloon 2. By changing the state of contraction and expansion of the balloon 2, the The stent is delivered to the lesion and disengaged from the stent. The stent includes a stent base 4 and a drug coating disposed on the stent base 4. In this embodiment, the material of the stent base is a biodegradable material, specifically polylactic acid. In this embodiment, the in vivo degradation time of the stent matrix 4 is 24-48 months, and the degradation time will vary depending on the physical condition of the patient. The melt flow index of the polylactic acid described in this example is 42045g / 10min, and the test conditions for the melt flow index are: GB / T3682-2000 190°C / 2.16Kg. The tensile strength of the polylactic acid is greater than or equal to 50MPa, and the test reference standard for the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Shore hardness | aaaaa | aaaaa |
| Shore hardness | aaaaa | aaaaa |
| Shore hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com