Preparation method of prohexadione calcium intermediate
A technology of prohexadione calcium and intermediates, which is applied in the field of preparation of prohexadione calcium intermediates, can solve the problems of low atom economy, many reaction steps and the like, and achieves the effects of easy industrialization, simple operation and convenient post-processing process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
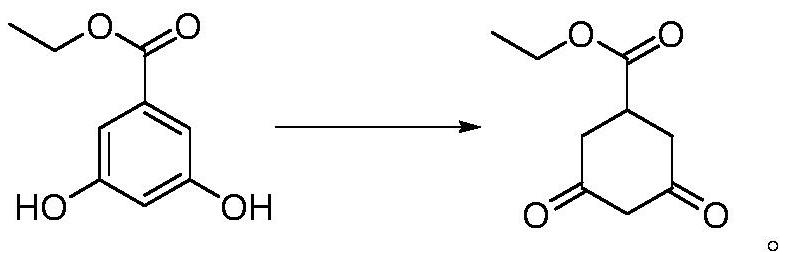
[0036] Add 182 grams of ethyl 3,5-dihydroxybenzoate into a 500ml stainless steel reaction kettle, add 182 grams of tetrahydrofuran, 92 grams of formic acid, add 1.82 grams of Raney nickel, close the reaction kettle, it cannot be opened during the process, and the temperature is raised to 100 ° C , and reacted for 10 hours. After the reaction was completed, it was cooled to 20 °C, 1.80 g of Raney nickel was recovered by filtration, and 180 g of tetrahydrofuran was recovered by rotary evaporation to obtain 176 g of ethyl 3,5-dioxohexylcarboxylate. The yield was 95.6%. HPLC Content 98.7%.
[0037] Product H NMR spectrum (CDCl3) δ: 1.2-1.3 (3H, CH 3 ),2.6-2.7(1H,CH),2.5-3.0(4H,CH2,CH2),3.58-3.62(2H,CH 2 ), 4.1-4.3 (2H,CH 2 ).
[0038] Elemental Analysis: Carbon 58.7%, Hydrogen 6.6%, Oxygen 34.7%.
[0039] Main fragment peaks MS(m / z): 184(M+), 139(M-OC2H5), 111(M-COOC2H5), 69(M-C6H11O2), 41(M-C7H11O3), 29(M-C8H10O5 )
[0040] Identified as ethyl 3,5-dioxohexylcarboxylate.
Embodiment 2
[0042] Add 182 grams of ethyl 3,5-dihydroxybenzoate to a 1500ml stainless steel reaction kettle, add 364 grams of methanol, 460 grams of formic acid, add 9.1 grams of Raney nickel, close the reaction kettle, it cannot be opened during the process, and the temperature is raised to 150 ° C , and reacted for 24 hours. After the reaction was completed, it was cooled to 30° C., 9.0 grams of Raney nickel was recovered by filtration, 360 grams of methanol and 300 grams of formic acid were recovered by rotary evaporation, and 176 grams of ethyl 3,5-dioxohexyl formate were obtained in a yield of 176 grams. 96.7%, HPLC content 98.0%.
Embodiment 3
[0044] Add 182 grams of ethyl 3,5-dihydroxybenzoate into a 1500ml stainless steel reactor, add 300 grams of ethanol, 100 grams of formic acid, add 5.0 grams of Raney nickel, close the reactor, and it cannot be opened during the process, and the temperature is raised to 120 ° C , reacted for 12 hours, after the reaction was completed, cooled to 25 ° C, 4.8 g of Raney nickel was recovered by filtration, and 290 g of ethanol was recovered by rotary evaporation to obtain 174 g of ethyl 3,5-dioxohexylcarboxylate with a yield of 95.6%. HPLC Content 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com