BmSPI39 mutant and application thereof
A mutant and site-directed mutation technology, applied in the field of genetic engineering and enzyme engineering, can solve the problems affecting the genetic transformation and industrial application of inhibitors, the mechanism of activity is not completely clear, and the research is limited.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
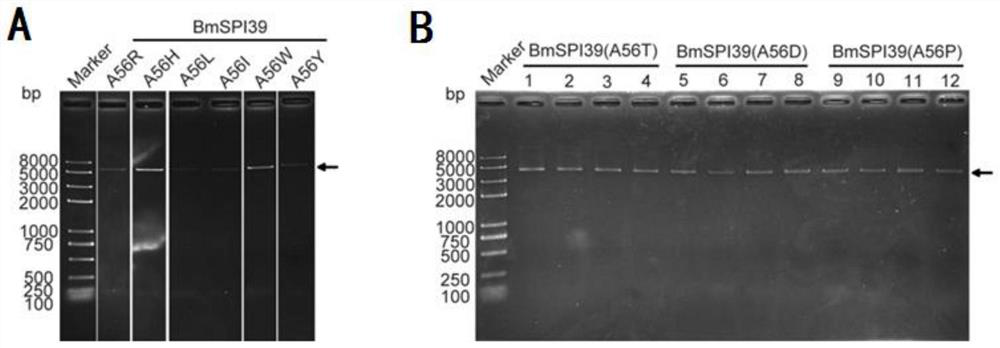
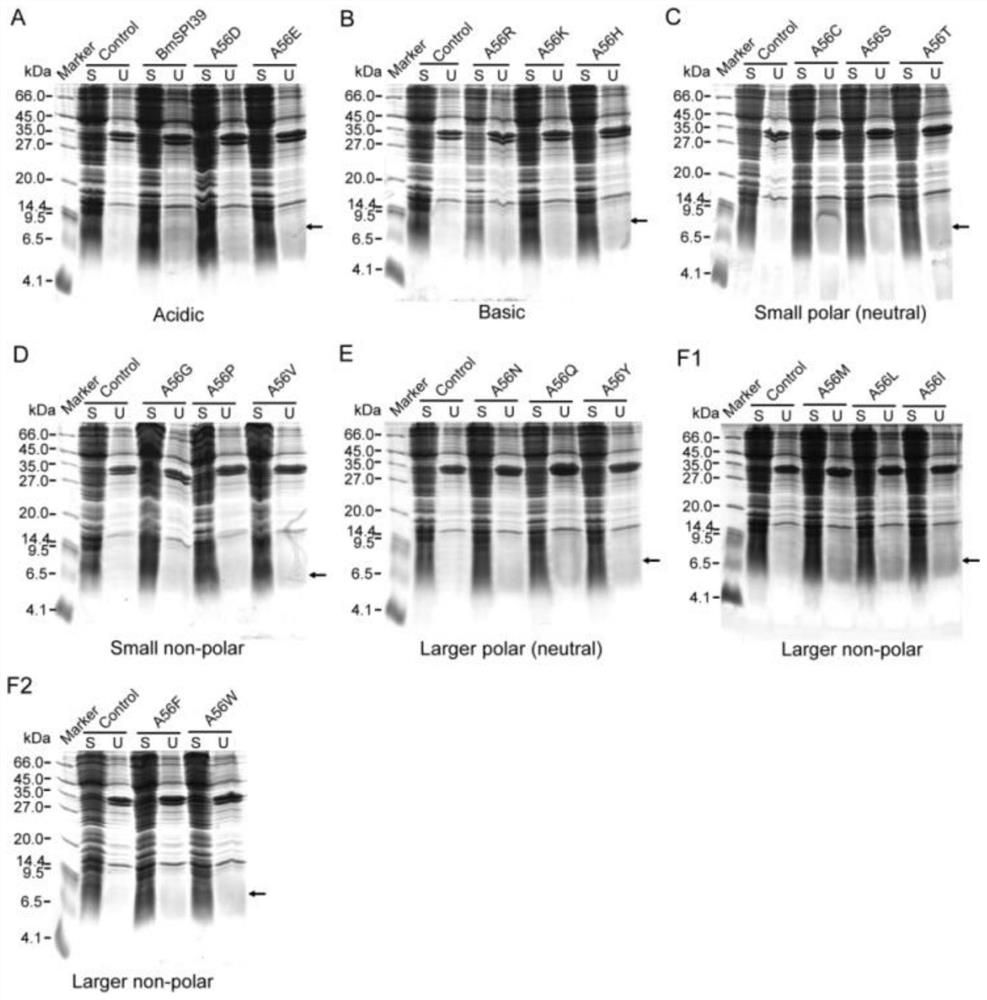
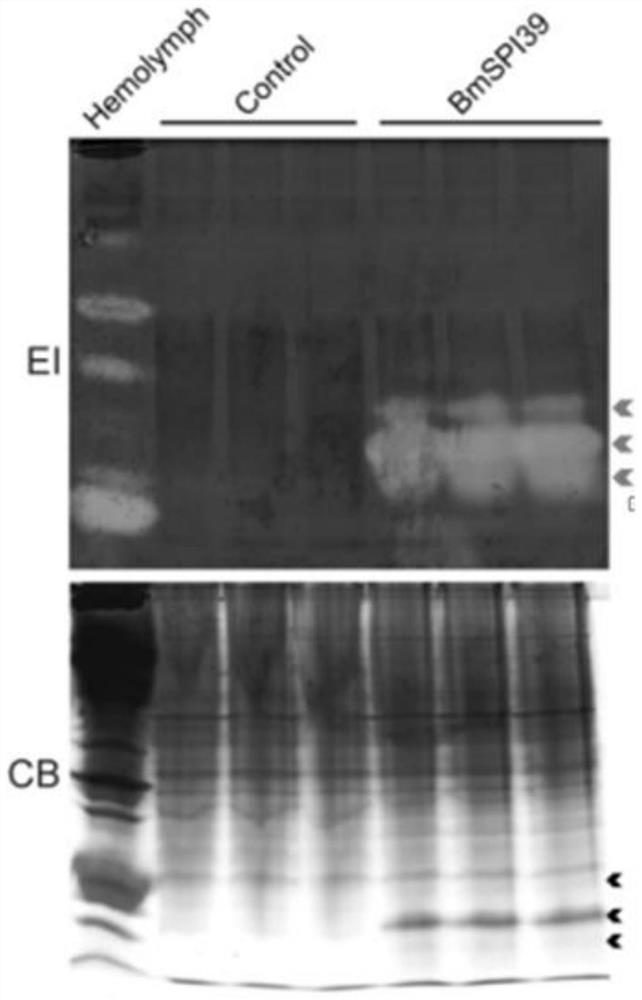
[0028] Construction and activity study of BmSPI39 mutant
[0029] 1. Mutation primer design
[0030] The amino acid sequence of BmSPI39 consists of positions 25 to 98 in SEQ ID NO.1, of which positions 1 to 24 are signal peptide sequences. Referring to the gene sequence of BmSPI39, as shown in SEQ ID NO.2, for BmSPI39 designed site-directed mutagenesis primers for 5' to 3' PCR amplification of this gene. The mutant template, desired mutation, DNA polymerase and primer sequences of BmSPI39 are shown in Table 1, respectively.
[0031]
[0032]
[0033] 2. PCR amplification
[0034] (1) When the DNA polymerase used in PCR amplification is FastPfu DNA Polymerase, the reaction system (25 μL) is shown in Table 2, and the amplification procedure is shown in Table 3. The amplification products were detected by 1% agarose gel electrophoresis.
[0035] Table 2 PCR reaction system
[0036]
[0037] Table 3 PCR amplification program
[0038]
[0039] (2) When the DNA poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com