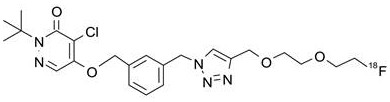
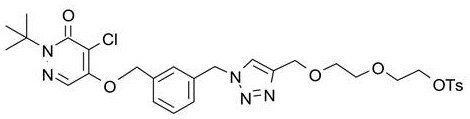
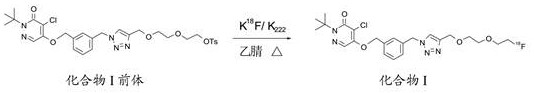
Liquid composition of compound I as well as preparation method and application of liquid composition
A technology for liquid compositions and compounds, applied in organic chemistry methods, chemical instruments and methods, introduction of isotopes into organic compounds, etc., can solve the problems of clinical application limitations, short half-life of imaging agents, etc., and improve radiochemical purity and stability. , the effect of improving radioactivity and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] In the prior art, amino polyether (K 222) is the high dilution method proposed by Lehn et al., which is one of the typical non-template ion synthesis methods. The specific steps are: 8-Diacyl chloride-3,6-dioxoctane was dissolved in a large amount of benzene solvent, and heated for 8 hours, then reduced by tetrahydroaluminum lithium for 24 hours, and then separated and recrystallized by column chromatography to obtain amino polyether (K 222 ). The method requires a large amount of solvent, such as benzene, the synthesis route is long, the operation is complicated, the yield is low, and the economic benefit is not high. In addition to the high dilution method, aminopolyether (K 222 ) is another classical synthesis method proposed by Kulstad and Malmsten using Na 2 CO 3 Equal to template in acetonitrile to obtain aminopolyether (K 222 ) sodium iodide complex, and then decomplexed by resin to obtain aminopolyether (K 222 ) synthesis method. The specific steps are a...
Embodiment 1
[0214] Example 1 Preparation of Compound I
[0215] 1) 18 Preparation of F ion solution
[0216] oxygenated [ 18 The water 2g of O] is transported to the accelerator target, and the accelerator is activated to generate a proton beam to bombard the oxygen-containing [ 18 O] water, producing a 18 The solution of F ions, this example uses a large dose to start 18 F, 18 The F initial activity is 3.5Ci.
[0217] 2) 18 F ion enrichment
[0218] prepared above 18 The solution of F ions is passed through an anion exchange solid phase extraction cartridge (Waters brand QMA cartridge, the QMA cartridge is preferably 1 mol / L NaHCO 3 activation), 18 F ions are enriched on the QMA cartridge.
[0219] 3) 18 F ion elution
[0220] Elution with cave ether and alkali metal salt catalyst solution, elution 18 F ions into the reaction flask, specifically, the K 222 15mg (dissolved in 0.9mL acetonitrile) with K 2 CO 3 1.5 mg (dissolved in 0.1 mL of water) was mixed to prepare a m...
Embodiment 2
[0234] The difference between Example 2 and Example 1 is only: 5) 8 In the nucleophilic substitution reaction of F ions, the amount of compound I precursor was 20 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com