Method for synthesizing imino benzotriazole compound under photocatalysis condition
A technology of imino benzotriazoles and benzotriazoles, which is applied in the field of compound preparation, can solve the problems of harsh reaction conditions and need metal catalysts, and achieves the effects of improving yield, improving protonation process and wide sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of N-ethyl acetate-1-benzotriazolyl-2-methylimine
[0038] A 10 mL Schlenk tube was added with a magnetic stirrer, followed by 0.4 mmol of benzotriazole (CAS: 95-14-7) and 1.2 mmol of ethyl diazoacetate (CAS: 623-73-4) , 1.6 mmol of methanol (CAS: 67-56-1), and finally 4 mL of acetonitrile (CAS: 75-05-8) was added.
[0039] By using a double-row tube, a 10 mL Schlenk tube was filled with argon; stirred with a magnetic stirrer, irradiated with a blue LED (445 nm) light and heated to 60 °C for 12 h, the final product was detected by TLC, and finally separated by column chromatography The final product N-ethyl acetate-1-benzotriazolyl-2-methylimine was obtained in 83% yield. The reaction equation is as follows:
[0040]
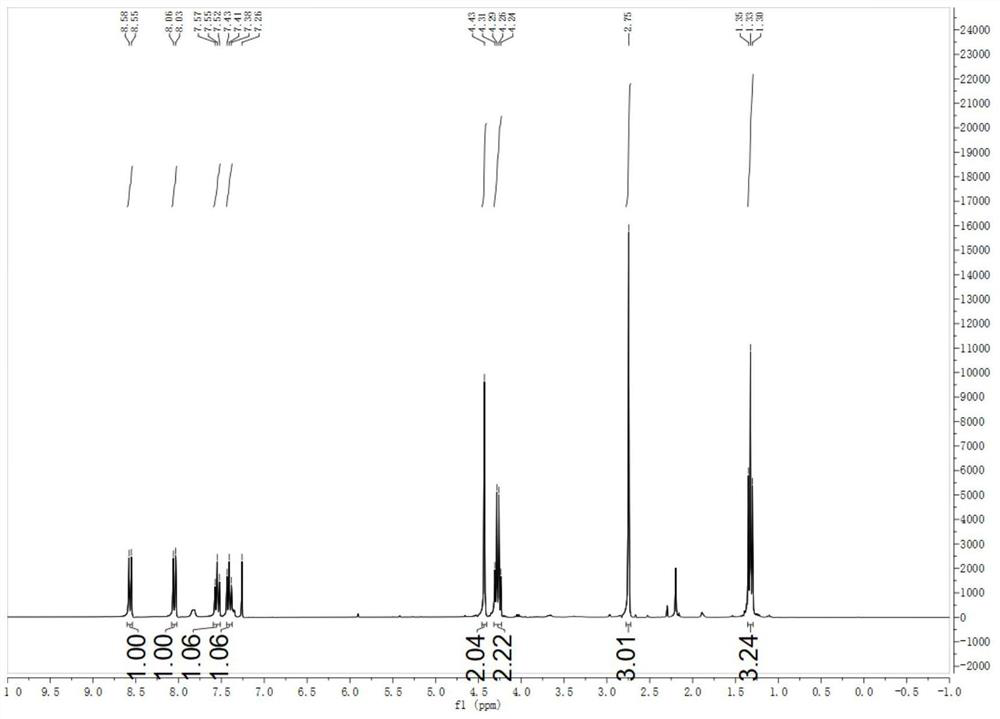
[0041] The NMR spectrum of N-ethyl acetate-1-benzotriazolyl-2-methylimine is characterized as follows:
[0042] 1 H NMR (300MHz, CDCl 3 )δ8.57(d,J=8.3Hz,1H),8.05(d,J=8.3Hz,1H),7.55(t,J=7.7Hz,1H),7.41(t,J=7.7Hz,1H) ,4.43(s,2H...
Embodiment 2
[0045] The preparation of embodiment two N-benzyl acetate-1-benzotriazolyl-2-methylimine
[0046]A 10 mL Schlenk tube was added with a magnetic stir bar, followed by 0.4 mmol of benzotriazole, 1.2 mmol of benzyl diazoacetate, 1.6 mmol of methanol, and finally 4 mL of acetonitrile.
[0047] By using a double-row tube, a 10 mL Schlenk tube was filled with argon; stirred with a magnetic stirrer, irradiated with a blue LED (445 nm) light and heated to 60 °C for 12 h, the final product was detected by TLC, and finally separated by column chromatography The final product N-benzyl acetate-1-benzotriazolyl-2-methylimine was obtained in 65% yield. (Benzyl diazoacetate is a reference synthesis (Photocatalyticgem-Difluoroolefination Reactions by a Formal C-C Coupling / Defluorination Reaction with Diazoacetates.Angew.Chem.Int.Ed.10.1002 / anie.202111892)) reaction equation is as follows:
[0048]
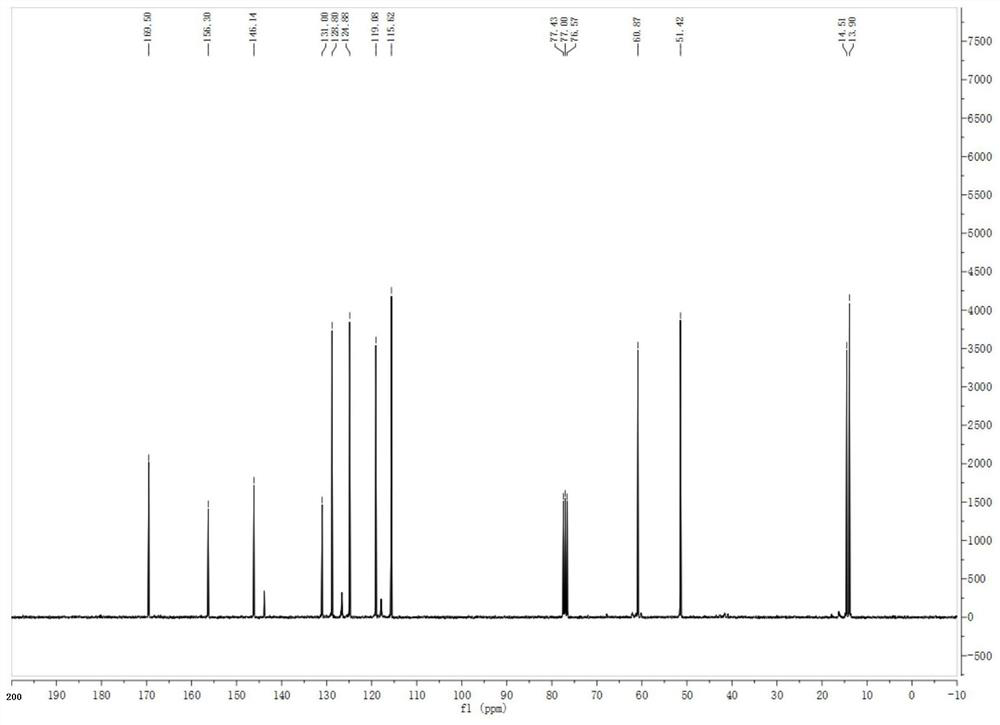
[0049] The NMR spectrum of N-benzyl acetate-1-benzotriazolyl-2-methylimine is characterize...
Embodiment 3
[0053] Example 3 Preparation of N-propynyl acetate-1-benzotriazolyl-2-methylimine
[0054] A 10 mL Schlenk tube was added with a magnetic stir bar, then 0.4 mmol of benzotriazole, 1.2 mmol of propynyl diazoacetate, 1.6 mmol of methanol, and finally 4 mL of acetonitrile.
[0055] By using a double-row tube, a 10 mL Schlenk tube was filled with argon; stirred with a magnetic stirrer, irradiated with a blue LED (445 nm) light and heated to 60 °C for 12 h, the final product was detected by TLC, and finally separated by column chromatography The final product N-propynyl acetate-1-benzotriazolyl-2-methylimine was obtained in 61% yield. (Propynyl diazoacetate is a reference synthesis (N,N'-Ditosylhydrazine:A Convenient Reagent for Facile Synthesis of Diazoacetates.Org.Lett.2007,9,3195-3197)) The reaction equation is as follows:
[0056]
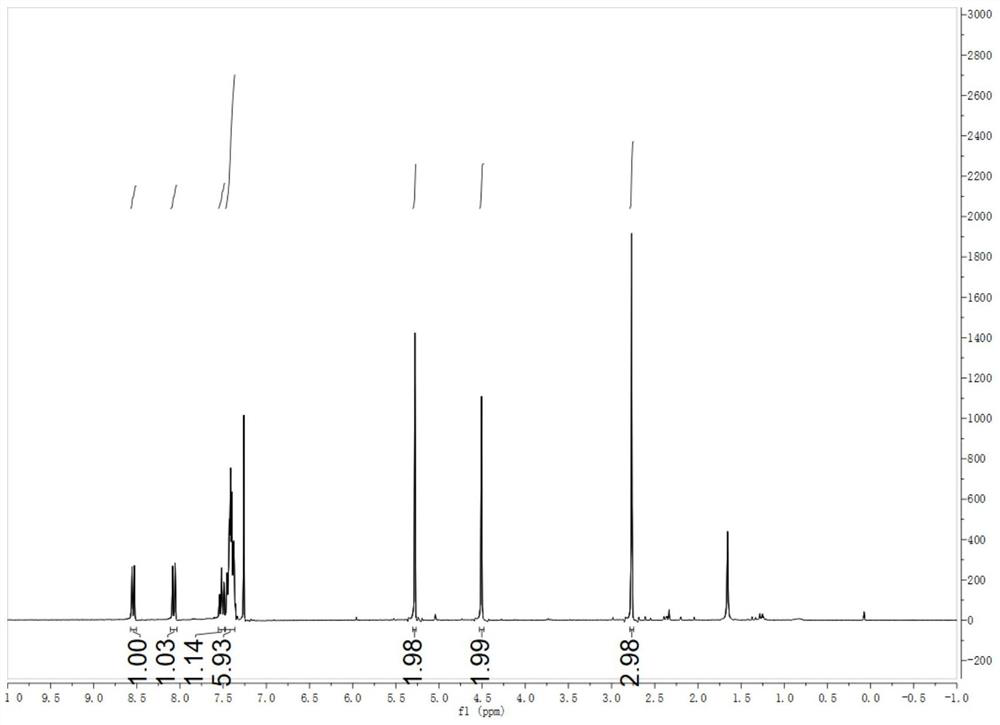
[0057] The H NMR characterization of N-propynyl acetate-1-benzotriazolyl-2-methylimine is as follows:
[0058] 1 H NMR (300MHz, CDCl 3 )δ8.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com