Carbazole quinoline heterocomplex as well as preparation method and application thereof
A carbazoquinoline and hybrid technology, which is applied in the field of carbazoquinoline hybrid fluorescent probes and their preparation, can solve the problems of uncomfortable and accurate detection, visualization of targets of interest and low signal-to-noise ratio, and achieves low The effect of background fluorescence interference and high tumor tissue fluorescence imaging selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: (E)-9-ethyl-3-(2-(quinolin-4-yl)vinyl)-9H-carbazole (I 1 ) preparation
[0037] 9-ethyl-9H-carbazole-3-carbaldehyde (500mg, 1.0mmol) and 4-methylquinoline (320mg, 1.0mmol) were added to a single-necked flask, dissolved in absolute ethanol (10ml), followed by adding 1 -2 drops of piperidine, refluxed at 85 °C for 12 h, after monitoring the reaction by TLC, suction filtration, recrystallization and purification to obtain compound I 1 , the yield is 85%.
[0038] (I 1 ) spectral data are: 1 H NMR (400MHz, DMSO-d 6 )δ9.39(d,J=6.3Hz,1H,ArH),9.15(d,J=8.5Hz,1H,ArH),8.78(s,1H,ArH),8.55–8.46(m,3H,ArH) ,8.42(m,2H,ArH),8.34(d,J=15.6Hz,1H,CH),8.26(m,1H,ArH),8.17(d,J=8.7Hz,1H,ArH),8.05(m , 2H, ArH), 7.84(s, 1H, ArH), 7.45(m, 1H, CH), 4.53(q, 2H, CH) 2 ),1.33(t,J=6.0Hz,3H,CH 3 ).
Embodiment 2
[0039] Example 2: (E)-9-ethyl-6-(2-(quinolin-4-yl)vinyl)-9H-carbazol-3-amine (I 2 ) preparation
[0040] Referring to Example 1 (I 1 ), the 9-ethyl-9H-carbazole-3-carbaldehyde in the method is replaced by 9-ethyl-6-nitro-9H-carbazole-3-carbaldehyde, and finally compound 4 is obtained; 4 (500mg, 1.0mmol), iron powder (273mg, 4.0mmol), ammonium chloride (523mg, 8.0mmol) were added to a single-neck bottle, dissolved in absolute ethanol (20ml), refluxed at 80°C for 5h, and the reaction was completed by TLC monitoring Then, suction filtration, the filtrate was spin-dried, and purified by column chromatography to obtain compound I2 , the yield was 72%.
[0041] (I 2 ) spectral data are: 1 H NMR (400MHz, DMSO-d 6 )δ9.35(d, J=6.8Hz, 1H, ArH), 9.12(m, 1H, ArH), 8.82(s, 1H, ArH), 8.58–8.44(m, 4H, ArH), 8.33(d, J=15.6Hz, 1H, CH), 8.24(m, 1H, ArH), 8.16(m, 1H, ArH), 8.02(m, 2H, ArH), 7.85(s, 1H, ArH), 7.41(m, 1H,CH),4.52(m,2H,CH 2 ),1.31(t,J=6.0Hz,3H,CH 3 ).
Embodiment 3
[0042] Example 3: Compound I obtained in Example 1 of the present invention 1 Ultraviolet absorption spectroscopy under different pH conditions
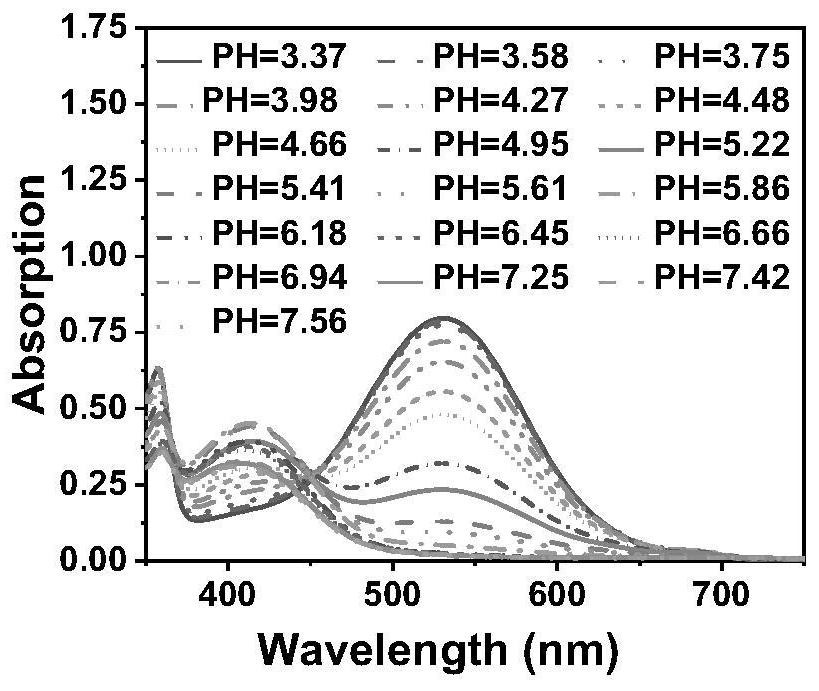
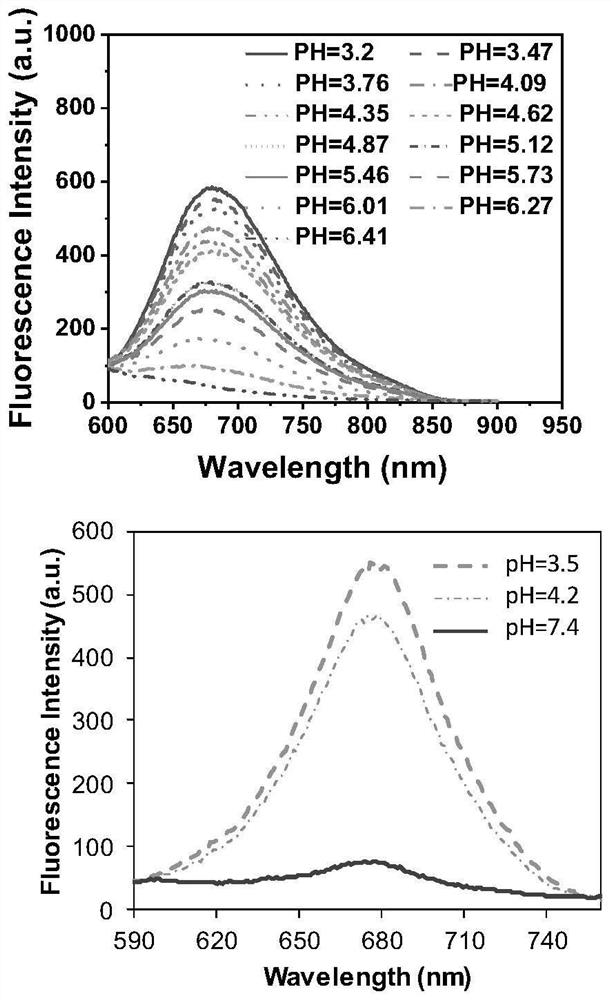
[0043] The fluorescent compound of the present invention is dissolved in an aqueous ethanol solution containing 50% to prepare a detection solution with pH=3-8 and a concentration of 20 μM. The UV-Vis spectrophotometer is used to test the UV absorption spectrum data, and the results show that the maximum UV absorption wavelength of the fluorescent compound of the present invention is in the range of 450-600 nm. wherein compound I 1 The UV absorption peak around 490 nm varies with compound I 1 On the contrary, its UV absorption peak around 530nm increases with the decrease of pH, and its peak value differs by 40 times ( figure 1 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com