Acetynoic acid and preparation method thereof
A technology of alkynoic acid and acetylene, applied in the field of alkynoic acid and its preparation, can solve the problems of long reaction time, increase the complexity of post-processing process, etc., achieve simple post-processing operation, high research value, economic and social environmental benefits, shorten the time The effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The present embodiment provides, the preparation method of phenylpropioic acid, comprises the following steps:
[0081] 1) In the autoclave, add 100 mg of dried 4A molecular sieves, 2 mmol of phenylacetylene, 2.4 mmol of cesium carbonate and 6 mL of anhydrous dimethyl sulfoxide.
[0082] 2) After sealing the autoclave, fill it with carbon dioxide gas, and then empty it. The replacement gas operation was repeated 6 times to ensure that no air remained in the reactor.
[0083] 3) Then charged with 0.1Mpa carbon dioxide, raised to 70°C and reacted for 20h.
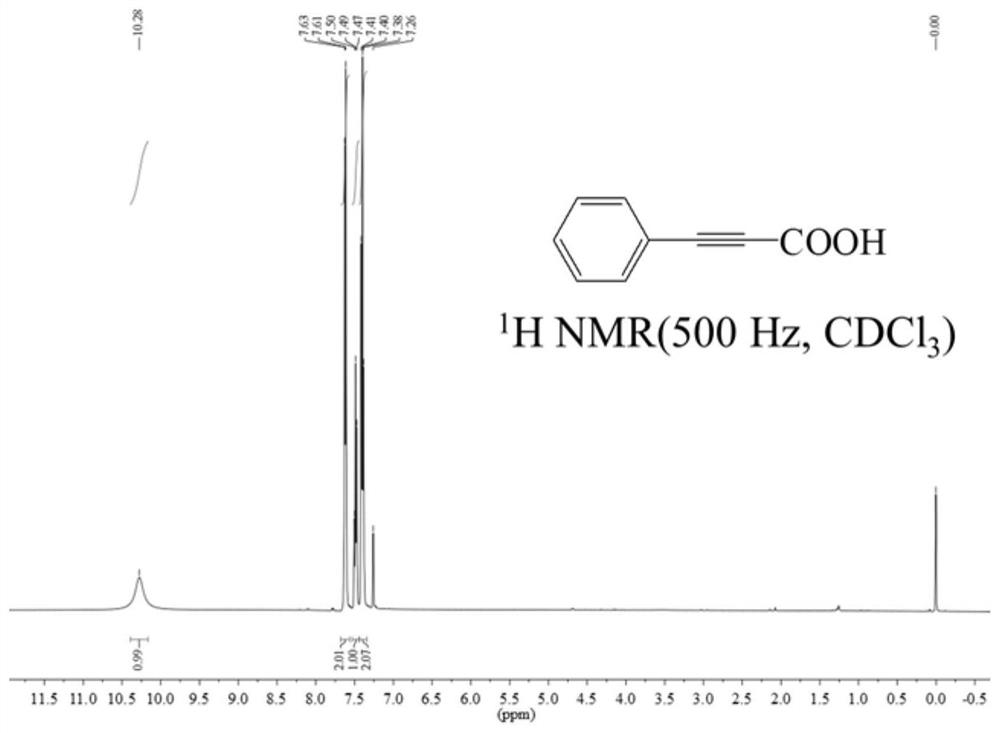
[0084] 4) After cooling the reaction product obtained in 3) to room temperature, remove the 4A molecular sieve, then add water with a volume ratio of 1:1 to dimethyl sulfoxide for mixing, continue stirring for 30 min, and extract 4 times with dichloromethane, The first aqueous phase was obtained; the first aqueous phase was cooled in an ice-water bath, acidified to pH 1 with HCl (V / V=1:1), and extracted four times wi...
Embodiment 2
[0088] The present embodiment provides, the preparation method of phenylpropioic acid, comprises the following steps:
[0089] 1) In the autoclave, add 100 mg of dried 4A molecular sieves, 2 mmol of phenylacetylene, 2.4 mmol of cesium carbonate and 6 mL of anhydrous dimethyl sulfoxide.
[0090] 2) After sealing the autoclave, fill it with carbon dioxide gas, and then empty it. The replacement gas operation was repeated 6 times to ensure that no air remained in the reactor.
[0091] 3) Then charged with 0.1Mpa carbon dioxide, raised to 40°C and reacted for 20h.
[0092] 4) After cooling the reaction product obtained in 3) to room temperature, remove the 4A molecular sieve, then add water with a volume ratio of 1:1 to dimethyl sulfoxide for mixing, continue stirring for 30 min, and extract 4 times with dichloromethane, The first aqueous phase was obtained; the first aqueous phase was cooled in an ice-water bath, acidified to pH 1 with HCl (V / V=1:1), and extracted four times wi...
Embodiment 3
[0095] The present embodiment provides, the preparation method of phenylpropioic acid, comprises the following steps:
[0096] 1) In the autoclave, add 100 mg of dried 4A molecular sieves, 2 mmol of phenylacetylene, 2.4 mmol of cesium carbonate and 6 mL of anhydrous dimethyl sulfoxide.
[0097] 2) After sealing the autoclave, fill it with carbon dioxide gas, and then empty it. The replacement gas operation was repeated 6 times to ensure that no air remained in the reactor.
[0098] 3) Then charged with 0.1Mpa carbon dioxide, raised to 50°C and reacted for 20h.
[0099] 4) After cooling the reaction product obtained in 3) to room temperature, remove the 4A molecular sieve, then add water with a volume ratio of 1:1 to dimethyl sulfoxide for mixing, continue stirring for 30 min, and extract 4 times with dichloromethane, The first aqueous phase was obtained; the first aqueous phase was cooled in an ice-water bath, acidified to pH 1 with HCl (V / V=1:1), and extracted four times wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com