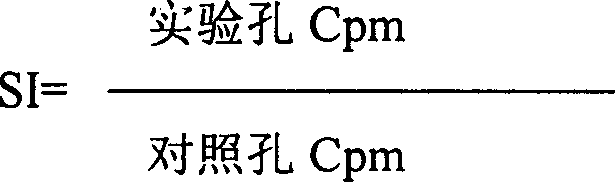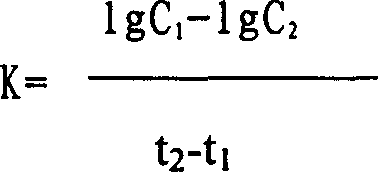Mannosan peptide and its prepn and use
A mannan peptide and composition technology, applied in the field of mannan peptide composition, can solve the problems of large oral dosage and complex ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] Experimental example 1, preparation of mannan peptide:
[0023] Fermentation process: i, the preparation of bacterial classification: select bacterial classification to be α-hemolytic streptococcus (Streptococcushemolyticus-α-hemolysis);
[0024] Culture medium: strain culture medium: glucose 0.4-0.8%, sodium chloride 0.4-0.8%, protein
[0025] Peptone 0.4-1%, beef extract 0.1-0.5%, sheep blood 10%, agar 1.5-2%,
[0026] PH7.2-7.8
[0027] Fermentation medium: glucose 0.1-0.5%, peptone 0.4-0.7%, yeast extract 0.4-0.6%,
[0028] Sodium chloride 0.4-0.8%, bubble enemy 0.01%
[0029] Raw and auxiliary materials required for extraction and refining processes: alcohol, acetone, trichloroacetic acid, absolute ethanol
[0030] a. Mother bottle preparation: Unseal the strain freezing tube, dilute it with sterile broth medium, and insert it into the blood slant under aseptic conditions. 18-45 ℃ constant temperature culture for 10-30h;
[0031] b...
experiment example 2
[0045] Experimental Example 2: Identification of Mannan Peptides
[0046] 1. Identification of strains: see the identification records of strains.
[0047] 2. Identification of semi-finished products:
[0048] i. Determination of absorbance: take an appropriate amount of semi-finished product sample, add water to make a solution containing 0.4mg in every 1ml, according to spectrophotometry (Chinese Pharmacopoeia 2000 edition two appendix VI A), at the wavelength of 260nm, its absorbance must not be greater than 0.25, at the wavelength of 280nm, its absorbance shall not be greater than 0.20.
[0049] ii. Content determination:
[0050] a. Preparation of reference substance solution Accurately weigh 0.1g of D-mannose reference substance dried at 105°C to constant weight, put it in a 100ml measuring bottle, add water to dissolve and dilute to the mark, shake well; precisely measure 5ml, put it in a 100ml In a measuring bottle, add water to the mark and shake well. Each 1ml cont...
experiment example 3
[0078] Experimental Example 3: Determination of the weight average molecular weight of the mannan peptide prepared in Experimental Example 1
[0079] 1. Instrument
[0080] Shimadzu high performance liquid chromatography (including LC-10AT pump, CTO-10A constant temperature column oven, DGU-14A degassing device, RID-10A differential refractive index detector); TSK-G4000PWXL (or Shodex Ohpak SB-804HQ) chromatographic column ; Longzhida interface, computer (built-in special GPC software for polysaccharides); 50ul syringe; Millipore ultrapure water device; 0.45um filter membrane column head filter, vacuum filtration device; analytical balance.
[0081] 2. Preparation of reagents and samples
[0082] 1. Ultrapure water
[0083] 2. Column storage solution: 0.05 sodium azide solution (NaN3) solution
[0084] 3. Mobile phase: 0.7% sodium sulfate solution (containing 0.02% NaN3)
[0085] 4. The test solution: take appropriate amount of raw materials respectively, add mobile phase ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| distribution coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com