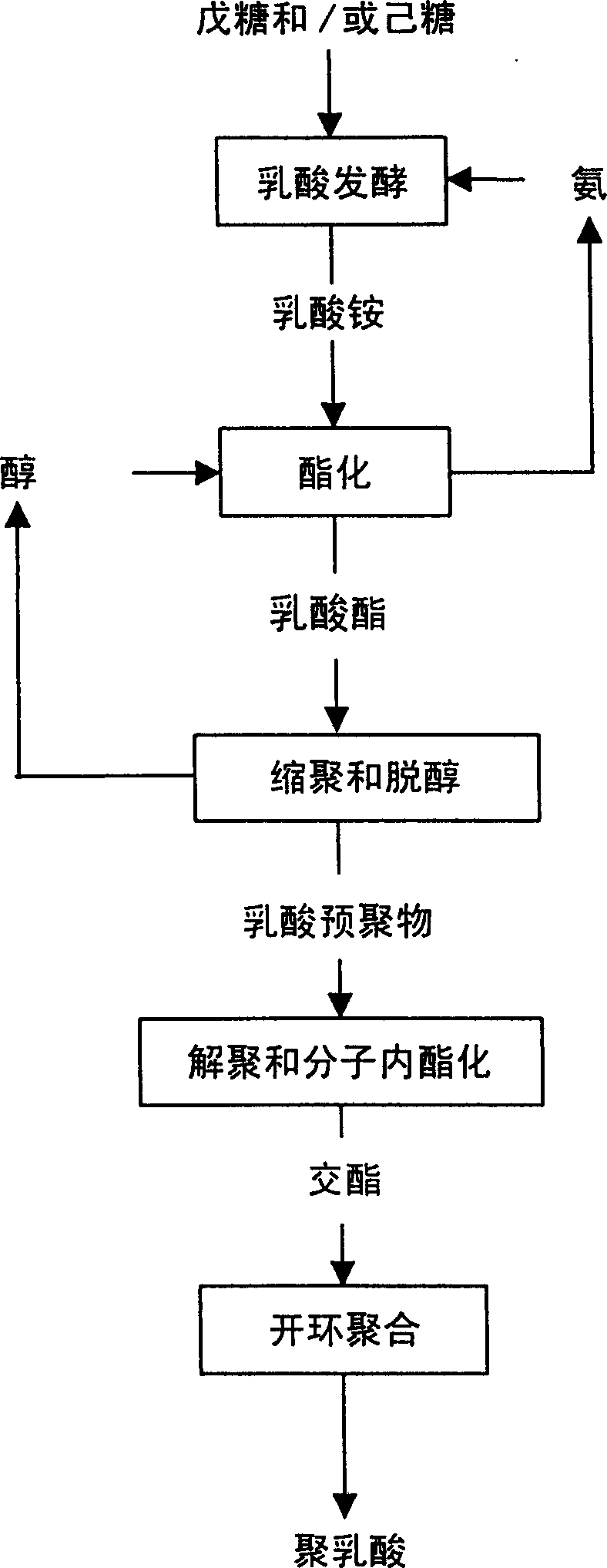
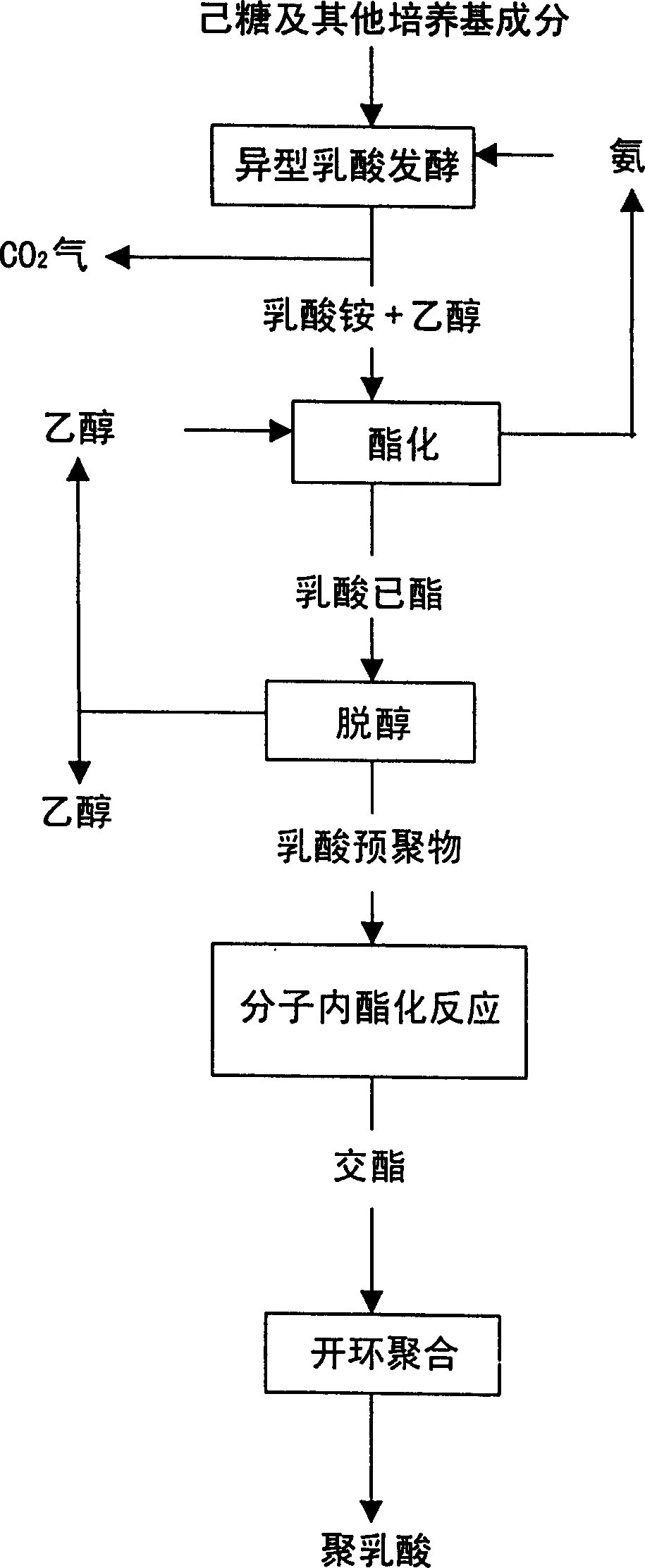
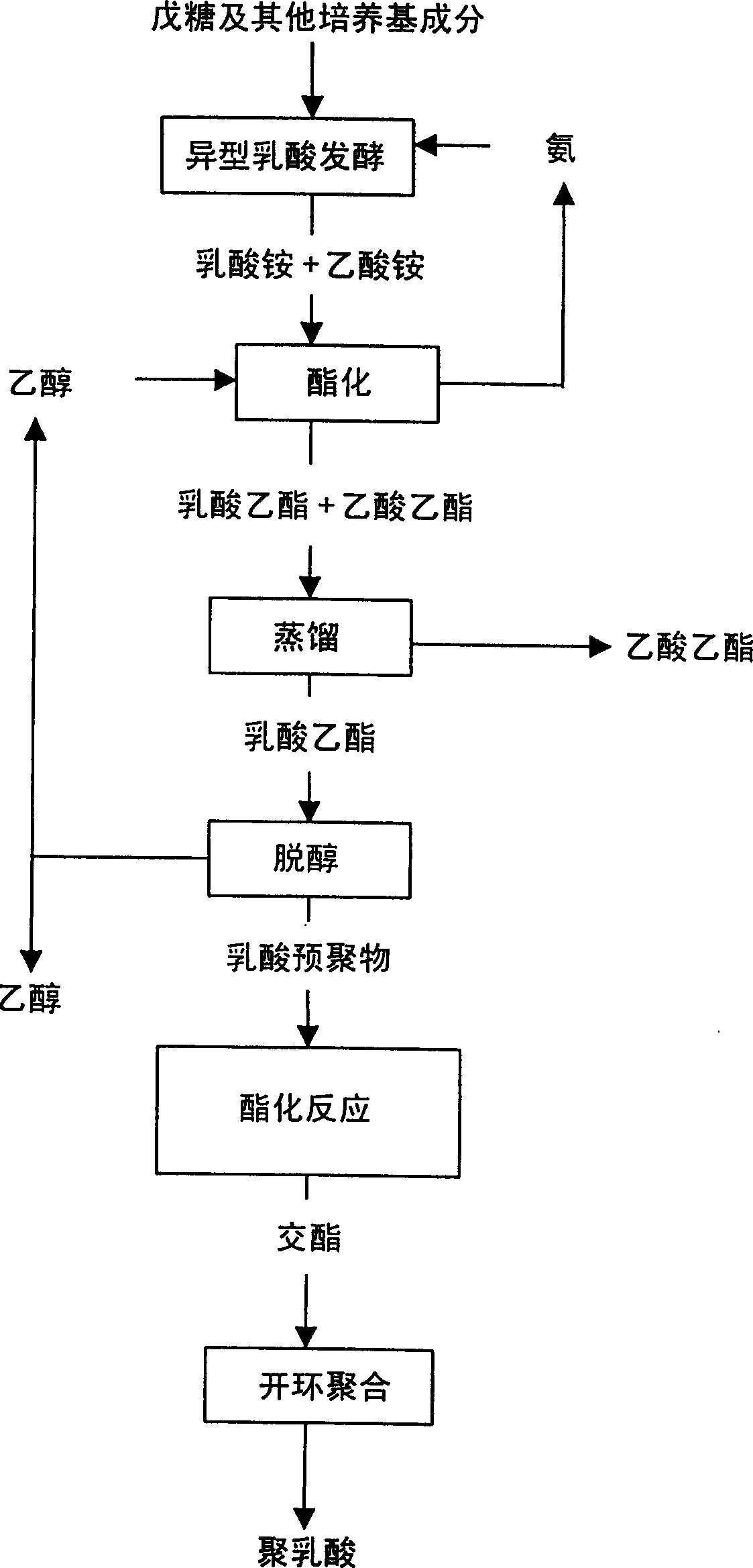
Mfg, Method of cyclic diester using fermented lactic acid as raw materila and method for mfg, polylacgtic acid
A manufacturing method and lactate technology are applied in the field of manufacturing polylactic acid and ammonium lactate to manufacture lactate, which can solve the problems of slow reaction and low yield, and achieve the effect of less investment, investment and economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] (1) Esterification and deamination process
[0081] In a flask with a volume of 1000ml with a stirrer, a cooling tube and a thermometer, 472g of L-ammonium lactate aqueous solution (68%) and 500g of ethanol are charged into a flask made by fermentation, the temperature of the reaction solution is maintained at 90-100°C, and the ethanol is refluxed For 3 hours, esterification proceeded. The ammonia that is not collected by the cooling pipe is recovered through the gas washing cylinder that is installed at the front of the cooling pipe and filled with ice water. Ammonia recovery was 98%.
[0082] (2) Removal of unreacted ethanol
[0083] The temperature of the reaction liquid obtained in (1) was kept at 80° C., and 360 g of unreacted ethanol was distilled off (recovered). Next, the temperature of the reaction liquid was raised to 120° C., and 150 g of water in the reaction liquid was distilled off.
[0084] (3) Refining process of ethyl lactate
[0085] The reaction ...
Embodiment 2
[0093] (1) Esterification and deamination process, (2) Unreacted ethanol removal process, (3) Ethyl lactate purification process were carried out exactly like Example 1.
[0094] (4) Ethyl lactate polycondensation process
[0095] In a 500ml flask with a stirrer, a reflux tube and a thermometer, 236g of the refined L-lactate obtained in (3) is charged, and the liquid temperature is raised to 160°C from 135°C in stages, and the pressure is changed from normal pressure Slowly depressurize for 3 hours to 5mmHg. During this period, L-lactic acid ethyl ester and the L-lactic acid oligomer produced by the polycondensation of L-ethyl lactate are refluxed in the flask, and in order to take the generated ethanol out of the system, the temperature of the cooling water in the reflux pipe is divided. Rise from 10°C to 60°C step by step. At this time, 91 g of ethanol produced was collected in a cold tank provided at the front of the return pipe. The weight-average molecular weight of th...
Embodiment 3
[0101] (1) Esterification and deamination process, (2) Unreacted ethanol removal process, (3) Ethyl lactate purification process were carried out exactly like Example 1.
[0102] (4) Ethyl lactate polycondensation process
[0103] In a 500ml flask with a stirrer, a reflux tube and a thermometer, 236g of refined L-lactate (optical purity 99.7%) obtained in (3) is charged, and the liquid temperature is gradually increased from 135°C under normal pressure. Rise to 220°C, during which L-lactic acid ethyl ester and L-lactic acid oligomers generated by the polycondensation of L-ethyl lactate are refluxed in the flask, and in order to take the generated ethanol out of the system, the reflux tube The cooling water temperature is set at 85°C.
[0104] Next, the pressure was gradually reduced from normal pressure to 5 mmHg for 3 hours. During this time, the L-lactic acid oligomer produced by the polycondensation of L-ethyl lactate was refluxed in the flask, and in order to take out th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com