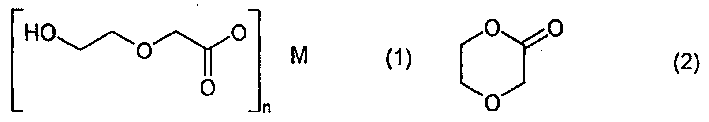Pure beta-hydroxyethoxyacetic acid salt, pure 2-p-dioxaneone, and prodn. method thereof
A kind of technology of hydroxyethoxy acetate and p-dioxanone, applied in the field of precursor of pure 2-p-dioxanone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] [Example 1 (separation and purification of β-hydroxyethoxy sodium acetate)]
[0136] (1) Synthesis of Ethylene Glycol Monosodium Salt
[0137] 1200 g of xylene, 409.7 g (6.60 mol) of ethylene glycol and 240.0 g (6.00 mol) of sodium hydroxide were mixed under stirring at 130-140° C. for 21 hours to carry out azeotropic distillation. After cooling to 25°C, the monosodium salt of ethylene glycol was obtained by filtration under reduced pressure. The yield was 494.2 g, 98.5% based on sodium hydroxide.
[0138] (2) Synthesis of β-hydroxyethoxy sodium acetate
[0139] 210.2 g (2.50 mol) of ethylene glycol monosodium salt obtained in the above operation (1) was added to 650 g of ethylene glycol under stirring and dissolved at 70-80°C. Then, over a period of 1 hour, 285.5 g (2.45 mol) of sodium monochloroacetate was added at the same temperature, followed by stirring at the same temperature for 2 hours to obtain 1145.0 g of a sodium β-hydroxyethoxyacetate solution in ethylen...
Embodiment 2
[0153] [Example 2 (synthesis of 2-p-dioxanone)]
[0154] 301.5g (1.50mol) of β-hydroxyethoxy sodium acetate obtained by the method of Example 1 was mixed with 500ml of methanol, and 151.0g (1.45mol) was added to the base with stirring at 30-35°C for 10 minutes. ) 35% hydrochloric acid. Then, after continuous stirring at 20°C for 20 minutes, generated water and methanol were distilled off at 50°C under reduced pressure. After removing sodium chloride by filtration, 125.7 g of distillate were obtained at 78-79° C. and a reduced pressure of 0.8 kPa (6 Torr).
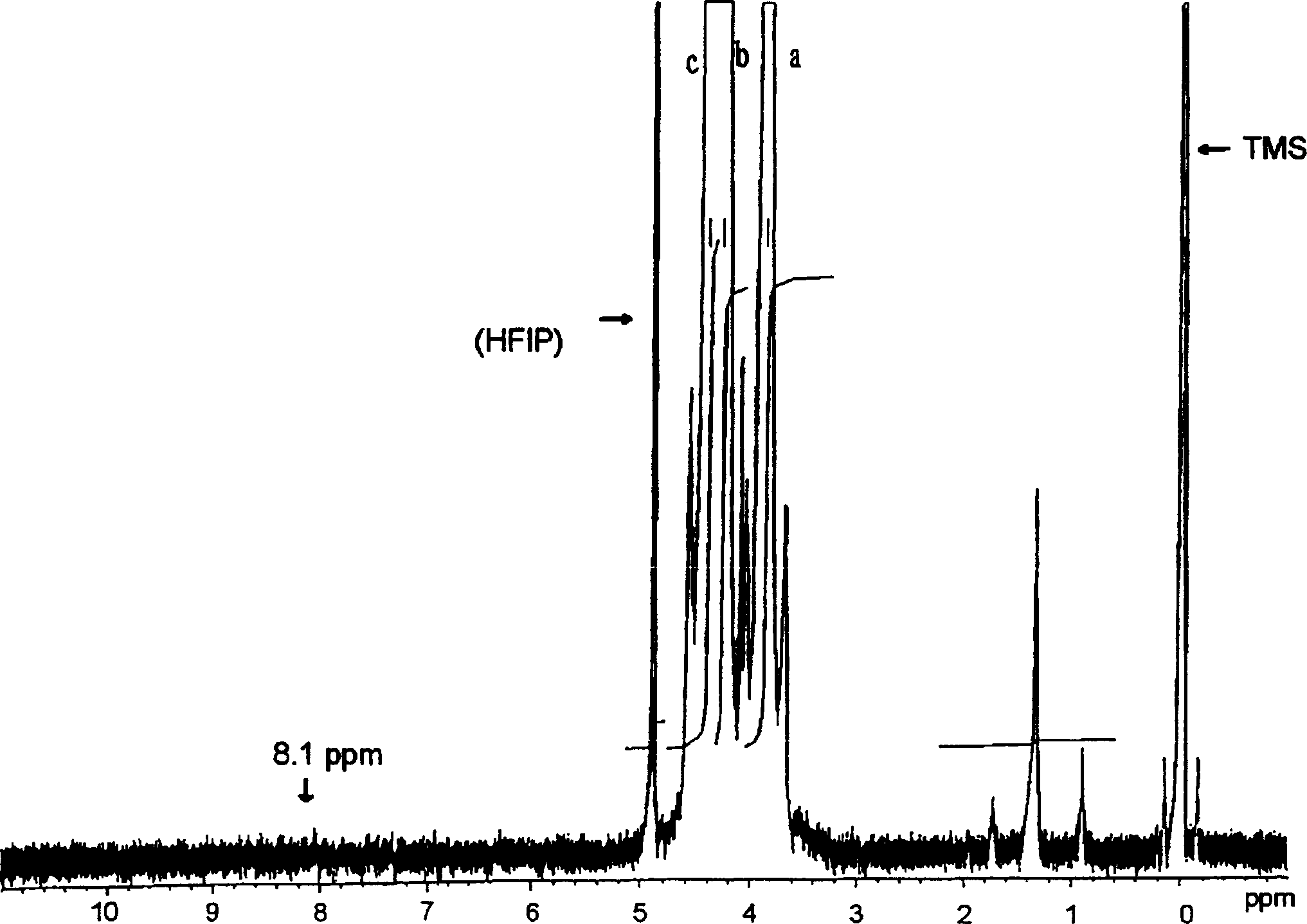
[0155] The results of NMR spectroscopy are as follows:
[0156] 1 H-NMR (400MHz, d 6 -DMSO,
[0157] δppm), 4.425(t, 2H, J=4.8Hz), 4.329(s, 2H), 3.832(t,
[0158] 2H, J=4.8Hz), 13 C-NMR (100MHz, d 6 -DMSO, δppm), 62.715,
[0159] 66.430, 69.430, 167.782,
[0160] It was confirmed to be 2-p-dioxanone.
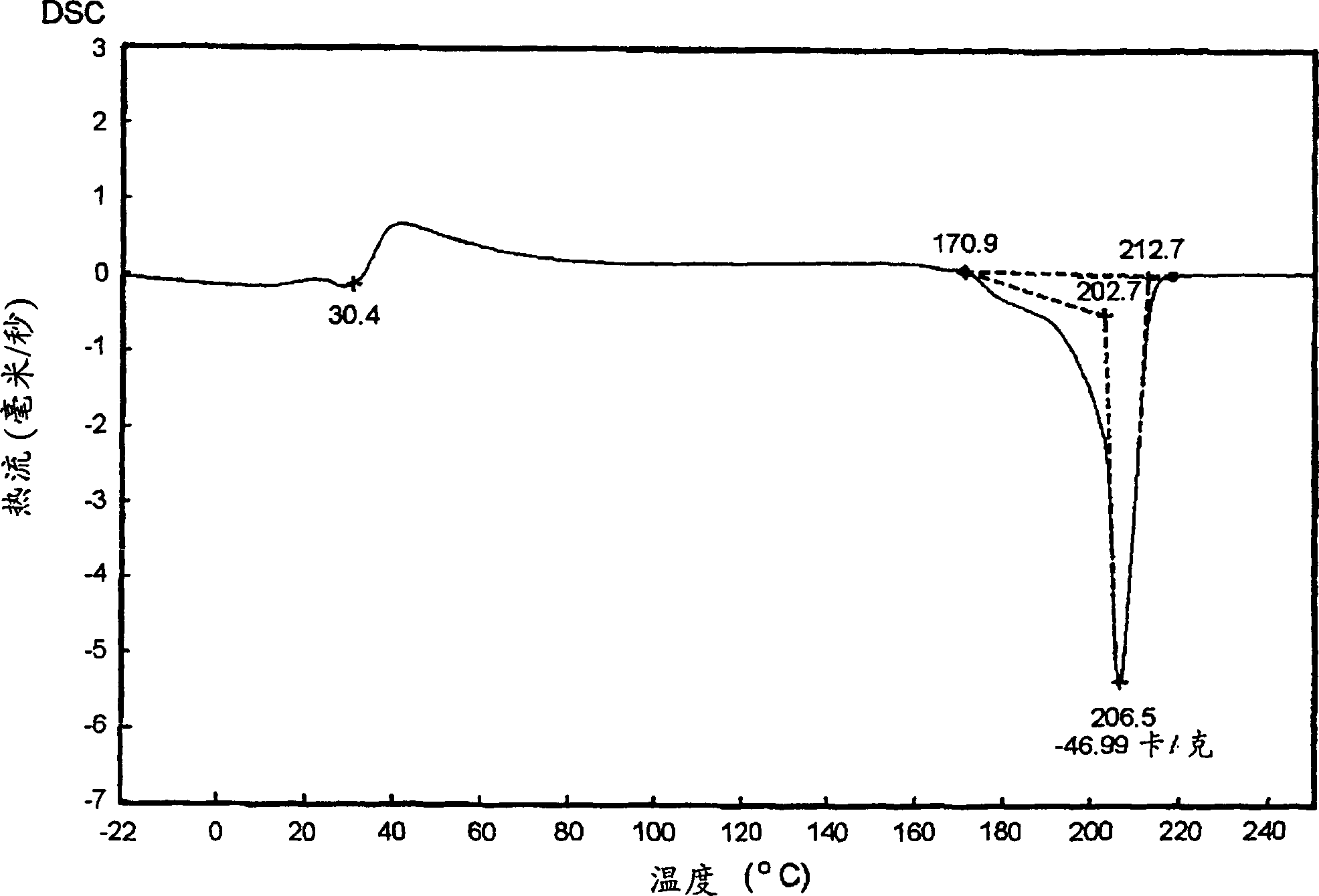
[0161] The melting point was 24.2° C., the purity determined by gas chromatography was 99.1%, and the yield base...
Embodiment 3
[0162] [Example 3 (synthesis of 2-p-dioxanone)]
[0163] 301.5g (1.50mol) of β-hydroxyethoxy sodium acetate obtained by the method of Example 1 was mixed with 500ml of dioxane, and 72.6g ( 0.73mol) 98% sulfuric acid. Then, stirring was continued at 20°C for 20 minutes, followed by removal of sodium chloride and sodium sulfate hydrate by filtration, at 78-79°C and a reduced pressure of 0.8 kPa (6 torr), to obtain 106.7 g of 2-p-dioxanone .
[0164] The melting point was 26.1° C., the purity determined by gas chromatography was 99.4%, and the yield based on sodium monochloroacetate was 56.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com