Process for production of pyridine derive
A kind of derivative and pyridine technology, applied in the field of preparation of pyridine derivatives, can solve the problems of high price, toxicity, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
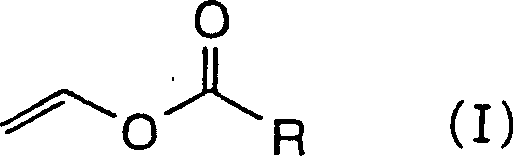
[0077] As the production method of the above-mentioned carboxylic acid vinyl esters, the direct vinylation method (J.Am.Chem.Soc., 69, pp.2439 (1947); J.Polymer.Sci., 1, pp.207 ( 1951); Trans.Faraday Soc., 49, pp.1108(1953); USPat.2,992,246 (1961); European Polymer J., 4, pp.373(1968)), vinyl exchange method (J.Org. Chem., 25, pp.623 (1960); Makromol. Chem., 73, pp.173 (1964); Makromol. Chem., 29, pp.119 (1959); J. Polymer Sci., 27, pp. 269(1958); Polymer Chemistry, 17, pp.227(1960); J.Sci.Eng.Res.Indian Inst.Technol.Kharagpur, 4, pp.265(1960)), Halcon method (J. Am. Chem. Soc., 81, pp. 2552 (1959)) and the like.
[0078] Carboxylic acid vinyl esters specifically include vinyl butyrate, vinyl caproate, vinyl caprylate, vinyl caprate, vinyl dodecanoate, vinyl myristate, vinyl palmitate, vinyl stearate, Vinyl cyclohexanecarboxylate, vinyl pivalate, vinyl octanoate, vinyl monochloroacetate, divinyl adipate, vinyl methacrylate, vinyl crotonate, vinyl sorbate, vinyl benzoate , v...
Embodiment
[0107] Next, the present invention will be described more specifically in conjunction with examples, but the present invention is not limited thereto. In addition, high performance liquid chromatography (hereinafter abbreviated as "HPLC") was used for evaluation of purity.
[0108] In addition, when "HPLC analysis" is described below, the measurement was performed under the following conditions, and when the conditions were changed, the conditions will be described in detail.
[0109] (Measuring conditions for HPLC analysis)
[0110] Column: YMC-A-312
[0111] Detection UV wavelength: 254nm
[0112] Eluent: acetonitrile / water=25 / 75, containing 0.2% by mass each of acetic acid and triethylamine as a buffer
[0113] Eluent flow rate: 1.0ml / min
Synthetic example 1
[0114] Synthesis example 1 (synthesis of raw material 3-(4-pyridyl)-1,2,4-triazine)
[0115] 200 ml of water, 200.0 g (1.92 mol) of 4-cyanopyridine, and 192.0 g (3.84 mol) of hydrazine monohydrate were charged into a 2000 ml four-necked flask, and reacted at 50° C. for 4 hours under stirring conditions. After confirming the disappearance of the raw material by HPLC analysis, 400 ml of toluene was added, and excess hydrazine monohydrate was distilled off, and this operation was repeated once more. 800 ml of water was added, followed by 278.4 g (1.92 mol) of 40% glyoxal aqueous solution, and the reaction was carried out at an external temperature of 100° C. for 2 hours. After the reaction was completed, it was cooled to 5° C. to obtain 222.4 g of the target product in the form of pale yellow crystals (yield 85.2%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com