Process for preparation of 3,4-methylenedioxy-mandelic acid
A technology of dioxyalmond and methylenedioxybenzene is applied in the field of preparation of 3,4-methylenedioxymandelic acid, and can solve the problems of adding amount recording and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
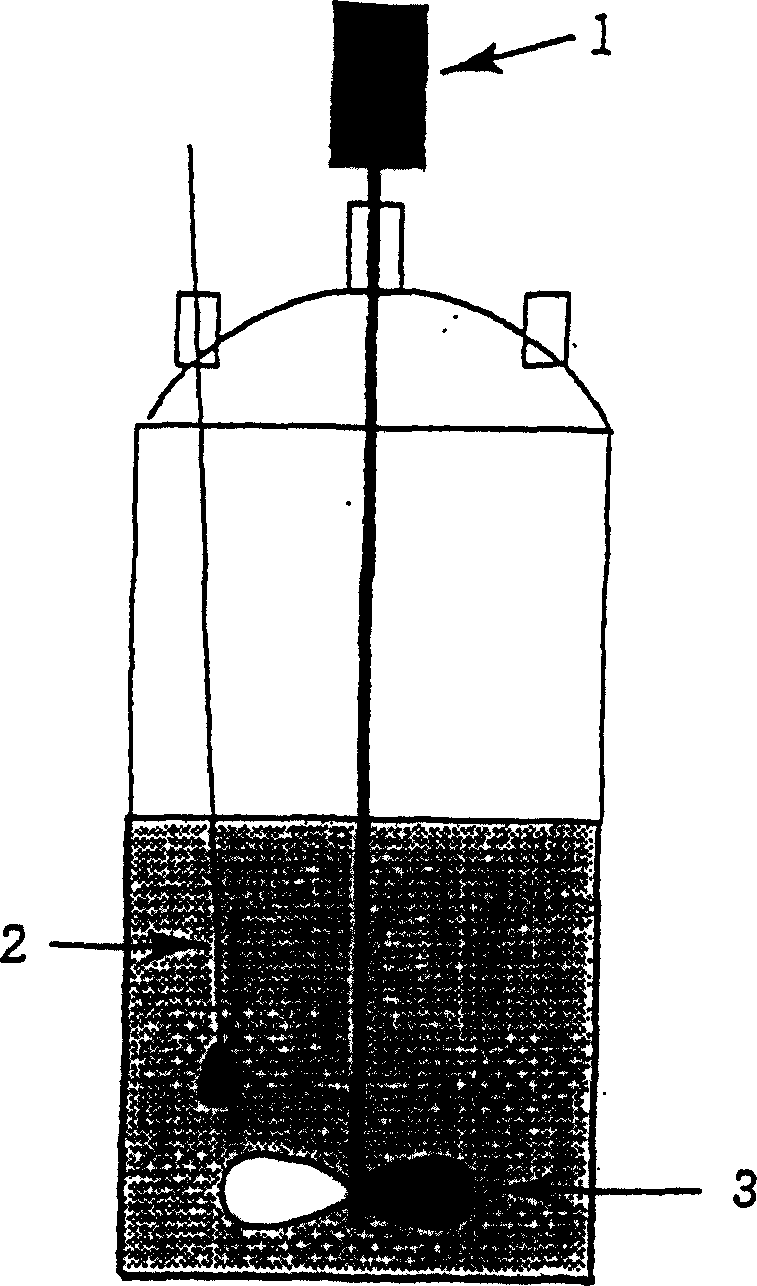
[0018] Under nitrogen atmosphere, to figure 1 Add 1,2-methylenedioxybenzene 50.0g (0.409mol) and 4-methyl-2-pentanone 25ml in the flat-bottomed split-neck flask of shown inner volume 500ml (organic solvent is relative to 1,2-methylene The amount of methylenedioxybenzene used is 500ml / kg), and then cooled to -5°C while stirring. in addition, figure 1 Among them, 1 represents the motor, 2 represents the thermometer, and 3 represents the stirring blade. Next, a mixed solution of 83.4 g (0.450 mol) of 40% by weight glyoxylic acid aqueous solution and 85.8 g (0.839 mol) of 96% by weight sulfuric acid was added dropwise, followed by stirring at -5°C for 21 hours. In addition, stirring during the reaction proceeded smoothly.
[0019] Then, 102.0 g (1.67 mol) of 28% by weight of ammonia water was slowly added for neutralization. Next, 100 ml of 2-butanone was added, heated to 60° C., and the produced 3,4-methylenedioxymandelic acid was extracted into the 2-butanone layer (organic ...
Embodiment 2~4
[0021] In Example 1, it reacted similarly to Example 1 except having changed the organic solvent used, reaction temperature, and reaction time. The results are shown in Table 1.
[0022]
Example
Aprotic organic solvents
temperature reflex
(℃)
Reaction time
(h)
MDB conversion
Rate(%)
MDMA selection
Selectivity (%)
2
-5
23
94
91
3
0
28
93
91
4
0
45
87
92
[0023] MDB: 1,2-Methylenedioxybenzene
[0024] MDMA: 3,4-methylenedioxymandelic acid
Embodiment 5
[0028] Under nitrogen atmosphere, to figure 1 Add 1,2-methylenedioxybenzene 500.0g (4.09mol) and 4-methyl-2-pentanone 250ml (organic solvent relative to 1,2-methylene dioxybenzene) The amount of methylenedioxybenzene used is 500ml / kg), and then cooled to -10°C while stirring. Next, a mixed solution of 833.7 g (4.05 mol) of 40% by weight glyoxylic acid aqueous solution and 857.5 g (8.39 mol) of 96% by weight sulfuric acid was slowly added dropwise, followed by stirring at -5°C for 23 hours. In addition, stirring during the reaction proceeded smoothly.
[0029] Then, 1030.0 g (16.93 mol) of 28% by weight of ammonia water was slowly added for neutralization. Next, add 3,000 ml of 4-methyl-2-pentanone, heat to 80°C, and extract the generated 3,4-methylenedioxymandelic acid into the 4-methyl-2-pentanone layer (organic layer) . The organic layer was analyzed by high performance liquid chromatography. As a result, the conversion rate of 1,2-methylenedioxybenzene was 95%, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com