Human erythropoietin Fc fusion protein with high bioactivity
A fusion protein, biologically active technology, applied in the fields of molecular biology and medicine, can solve the problems of prolonging EPO derivatives and having no half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Construction of the gene encoding the HuEPO-L-vFc fusion protein
[0025] 1.1 Construction of encoding HuEPO-L-vFc γ2 fusion protein gene
[0026] Fusion proteins are assembled from several DNA fragments. To obtain genes encoding the leader peptide and mature protein of human EPO, a human kidney cDNA library (obtained from Invitrogen, Carlsbad, CA) was used as a template for polymerase chain reaction (PCR). To facilitate cloning, SEQ ID NO: 1 introducing a restriction enzyme endonuclease site (HindIII) was used as a primer for the 5' oligonucleotide. Table 1 lists the oligonucleotide sequences used to clone the HuEPO-L-vFc fusion protein.
[0027] SEQ ID NO: 1
5'-cccaagcttggcgcggagatgggggtgca-3'
SEQ ID NO: 2
5'-cggatccgtcccctgtcctgcaggcct-3'
SEQ ID NO: 3
5'-gagcgcaaatgttgtgtcga-3'
SEQ ID NO: 4
5'-ggaattctcatttacccggagacaggga-3'
SEQ ID NO: 5
5'-tggttttctcgatggaggctgggaggcct-3'
SEQ ID NO: 6
...
Embodiment 2
[0041] Example 2. Expression of Fusion Proteins in Transfected Cell Lines
[0042] The recombinant pEFP1, pEFP2 or pEFP4 expression vector plasmids were transfected into mammalian host cell lines to express the HuEPO-L-vFc fusion protein. For stable high-level expression, a preferred host cell strain is a CHO cell deficient in the DHFR enzyme (see, eg, US Patent No. 4,818,679). A preferred transfection method is electroporation. Other methods can also be used, including calcium phosphate co-precipitation, lipofection, and protoplast fusion. In electroporation, 2-5 × 10 7 Add 10 μg of plasmid DNA linearized with BspCI to each cell. Two days after transfection, the medium was changed to growth medium containing 0.18 mg / ml G418. Transfectants resistant to selected drugs were tested for secretion of the fusion protein using an anti-human IgG Fc ELISA. Quantification of the expressed fusion protein can also be performed by ELISA using an anti-HuEPO assay. Wells producing high...
Embodiment 3
[0045] Example 3. Purification and characterization of fusion proteins
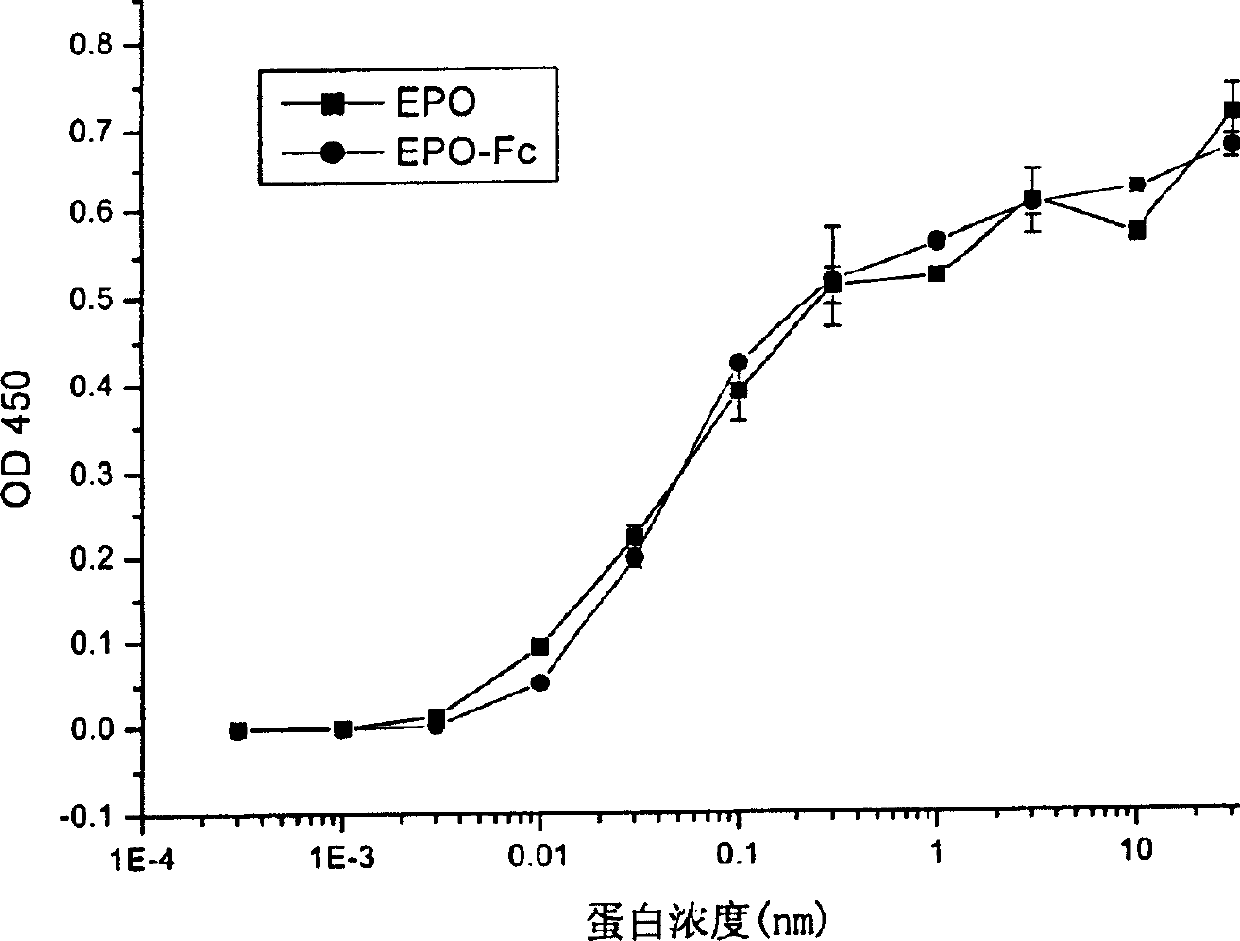
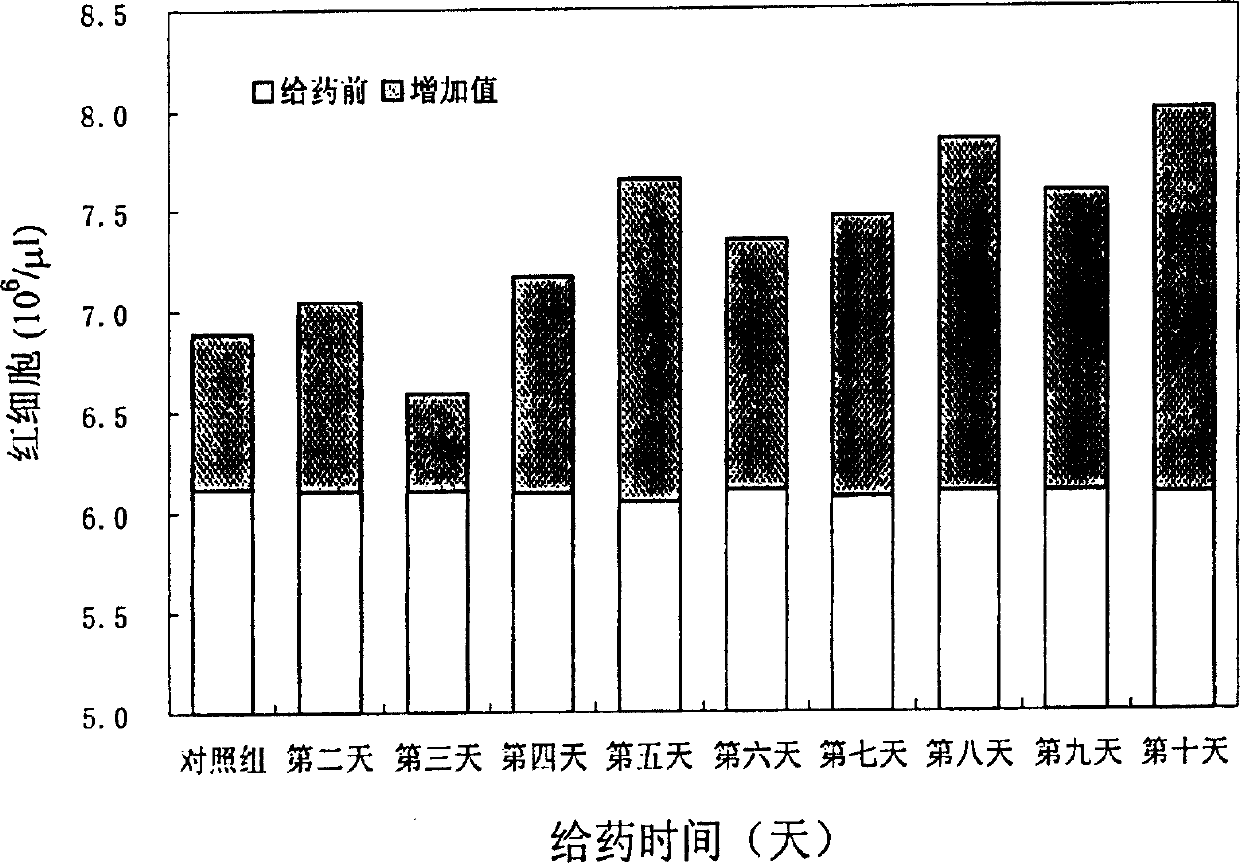
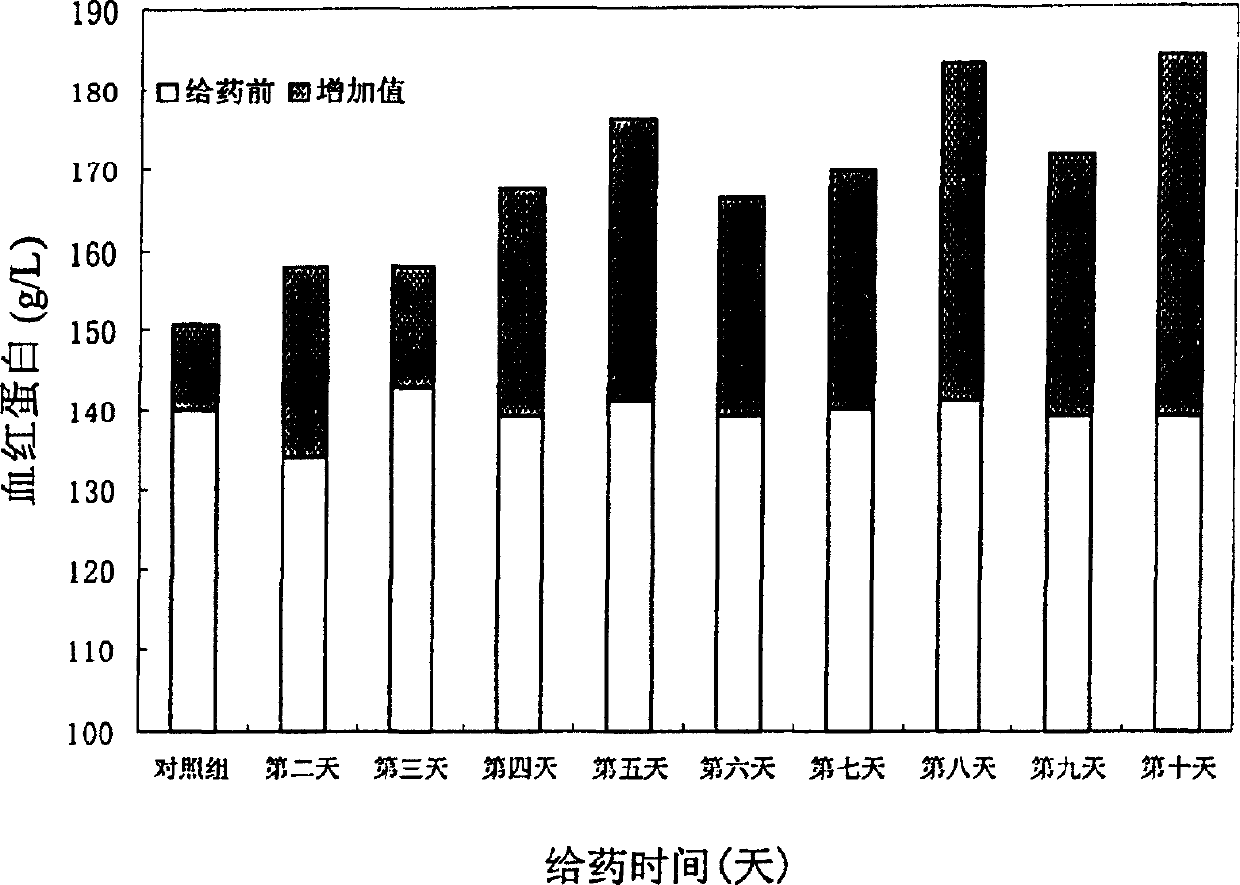
[0046] The conditioned medium containing the fusion protein was titrated to pH 7 to 8 with 1 N NaOH and filtered through a 0.45 micron nitrocellulose filter. The filtrate was loaded onto a Prosep A column equilibrated with phosphate buffered saline (PBS). After the fusion protein is bound to Prosep A, discard the flow-through fraction. The column was washed with PBS until the OD value at 280nm was below 0.01. The bound fusion protein was then eluted with 0.1 M citrate buffer, pH 3.75. with 0.4 volumes of 1M K 2 HPO 4 For neutralization, fractions containing purified protein were pooled and dialyzed against PBS. The solution was then filtered through a 0.22 micron nitrocellulose filter and stored at 4°C. Under non-reducing conditions, the molecular weight of the purified HuEPO-L-vFc protein ranged from 120 to 140 kDa as determined by SDS-PAGE. Under reducing conditions, the purified protein migrates...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com