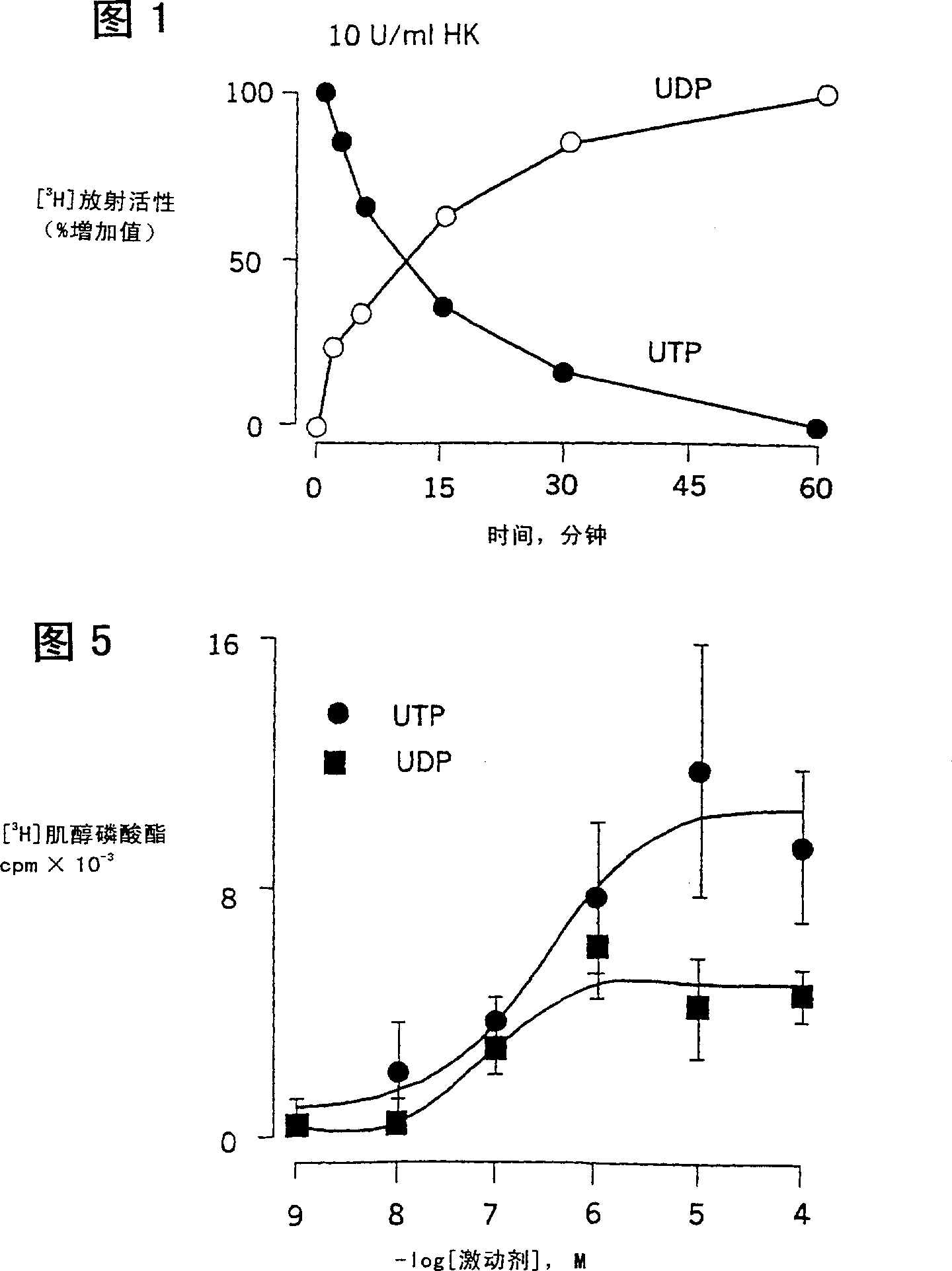
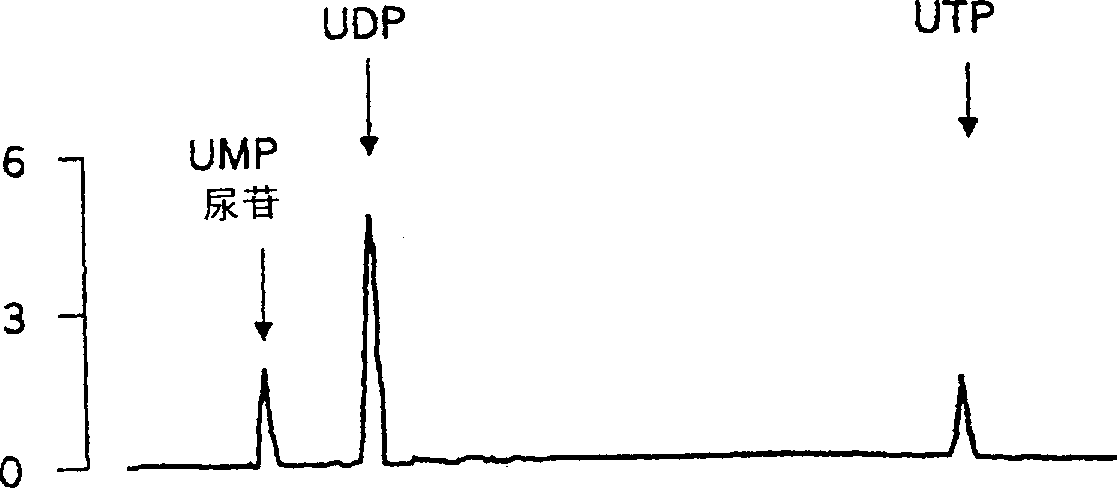
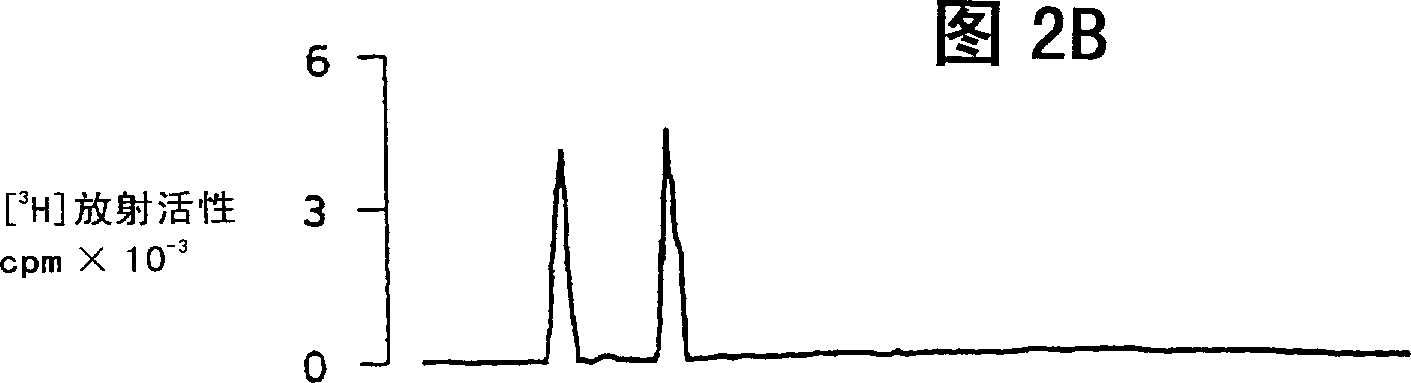
Use of uridine 5'-diphosphate and analogs thereof for the treatment of lung diseases
A technology of compounds and medicinal salts, applied in the direction of respiratory diseases, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as not realizing that it is useful for treating tracheal diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Method: Cell Culture
[0060] Human nasal epidermal cells were harvested from turbinates using protease XIV (Sigma, St.Louis, MO) at 4°C for 24-48 hours as previously described, R.Wu, et al., American Review of Respiratory Diseases (Am.Rev Respir. Dis.) 132, 311-320 (1985). Cytosolic calcium ions and inositol phosphate assays were performed on porous Transwell Col filters (pore diameter 0.45 μm; Costar, Cambridge, MA) maintained in Ham's F12 medium supplemented with 10 ng / mL epidermal growth factor, 3.75ng / mL epidermal growth factor, 500ng / mL hydrocortisone, 5ng / ml insulin, and 1mM CaCl 2 (Ham's F12+4X matrix). The assay is performed on days 7-10 after inoculation, which is the time coincident with the development of the maximum transepidermal potential difference according to N. Nakahata and T. K. Harden, Biochem. J. 241, 337-344 (1987).
Embodiment 2
[0062] Method: Determination of inositol phosphate
[0063] The fusion human nasal epithelial cells described in Example 1 were mixed with 4.5 g / L glucose and 5 μCi / mL[ 3 H] Inositol labeling in inositol-free DMEM for 18 hr. The cells were pre-incubated with 10 mM lithium chloride and challenged with agonist for 10 minutes. To prepare the same matrix, add [ 3 H] myo-inositol to avoid the release of endogenous ATP by stressed cells. E.R. Lazarowski et al., Br. J. Pharmacol 116, 1619-1627 (1995). Add 5% ice-cold trichloroacetic acid to stop the incubation, and separate the obtained [ 3 H] Inositol phosphate, according to the method described by A.M. Paradiso et al., Nature (Nature) 377, 643-646 (1995).
Embodiment 3
[0065] Methods: Bioelectricity and Cytosolic Ca 2+ Joint determination of
[0066] Nasal cells grown on Transwell Col filters with O-rings maintained in F12+4X matrix were studied 7-10 days after plating cells as described above. for Ca 2+ i Assay, load cells with Fura-2, then place the cells in a micro-Ussing chamber on a microscope (Zeiss) objective lens connected to a microfluorometer, and measure cytosolic Ca according to the method described in Paradiso et al., above. 2+ i content. Collect the fluorescence intensity ratio (excitation wavelength 340 / 380; emission wavelength λ450nm) from the area of 30-40 nasal cells on the monolayer surface, and then convert according to the method of E.H.Larsen et al., J.Physiol (J.Physiol) 424, 109-131 (1990) into Ca 2+ concentration. For the simultaneous determination of Cl - For the secretion, the transepithelial potential difference (TEP) was measured by Voltage-Clamp / Pulse Generator (Model VCC600, Physiological In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com