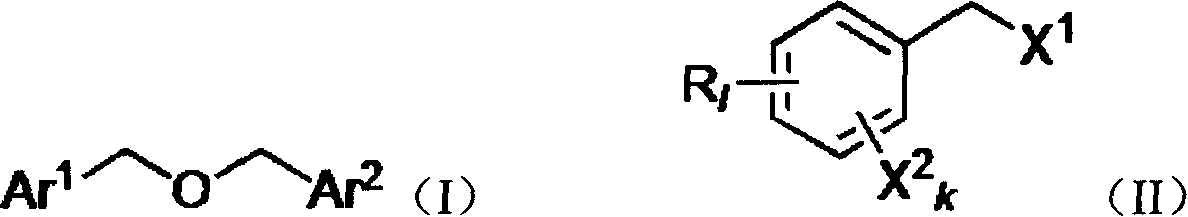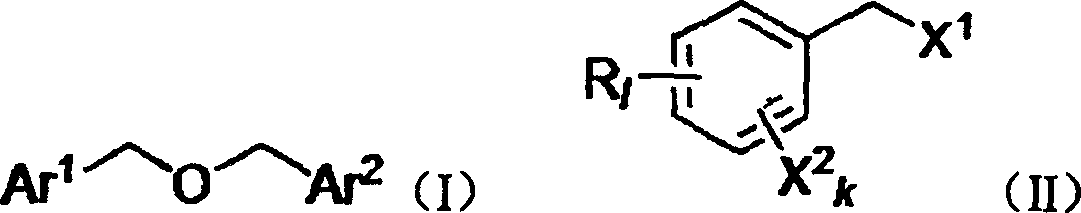Method for preparing substituted dibenzyl ether
A technology of dibenzyl ether and benzyl halide, which is applied in the field of preparation of substituted dibenzyl ethers, can solve the problems of lack of competitiveness, consume a large amount of alkali, and not involve the preparation method of substituted dibenzyl ethers, and reduce resource consumption , The effect of reducing waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of dibenzyl ether by hydrolysis of benzyl chloride
[0030] Take 2.53g of chlorobenzyl chloride, 0.10g of KI, and 18mL of water into the Erlenmeyer flask, quickly heat to 115℃, start refluxing, stir vigorously, reflux for 48 hours, the reaction pressure is 5 MPa, TLC detection (ethanol-n-hexane, 1:5), the composition no longer changes. The temperature was lowered to about 60°C, 20mL of toluene was added for extraction, the temperature was approaching room temperature, and the liquid was separated. The aqueous phase is extracted with ethyl acetate (10 mL×2) to recover hydrogen halide; the organic phases are combined, dried over anhydrous sodium sulfate, and evaporated to dryness (if a small amount of water remains, 10-20 mL of toluene can be added and evaporated to dryness). After passing through a silica gel column (the method and parameters of passing the silica gel column are known to those of ordinary skill in the art), 1.25 g of a colorless oily p...
Embodiment 2
[0031] Example 2: Preparation of dibenzyl ether by hydrolysis of benzyl bromide
[0032] The method of Example 1. But the raw material benzyl bromide (3.42g) was reacted for 24 hours. 1.33 g of colorless oily product was obtained, and 0.78 g of raw material was recovered.
Embodiment 3
[0033] Example 3: Preparation of 4,4′-dichlorobenzyl ether by hydrolysis of 4-chlorobenzyl chloride
[0034] The method of Example 1. But the raw material 4-chlorobenzyl chloride (3.22g), KI (0.17g), reacted for 24 hours. 1.70 g of colorless needle crystal product was obtained, and 0.91 g of raw material was recovered.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com