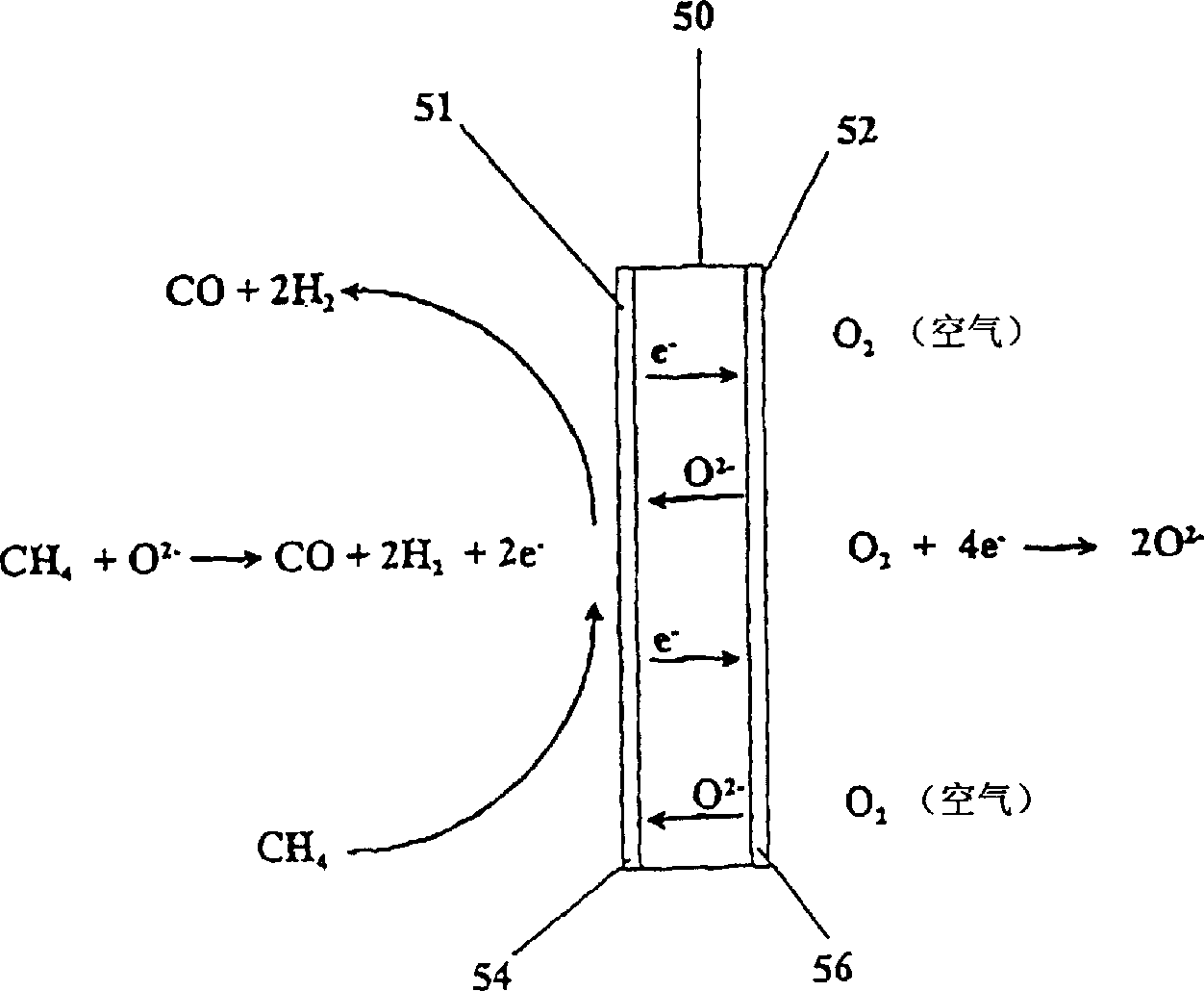
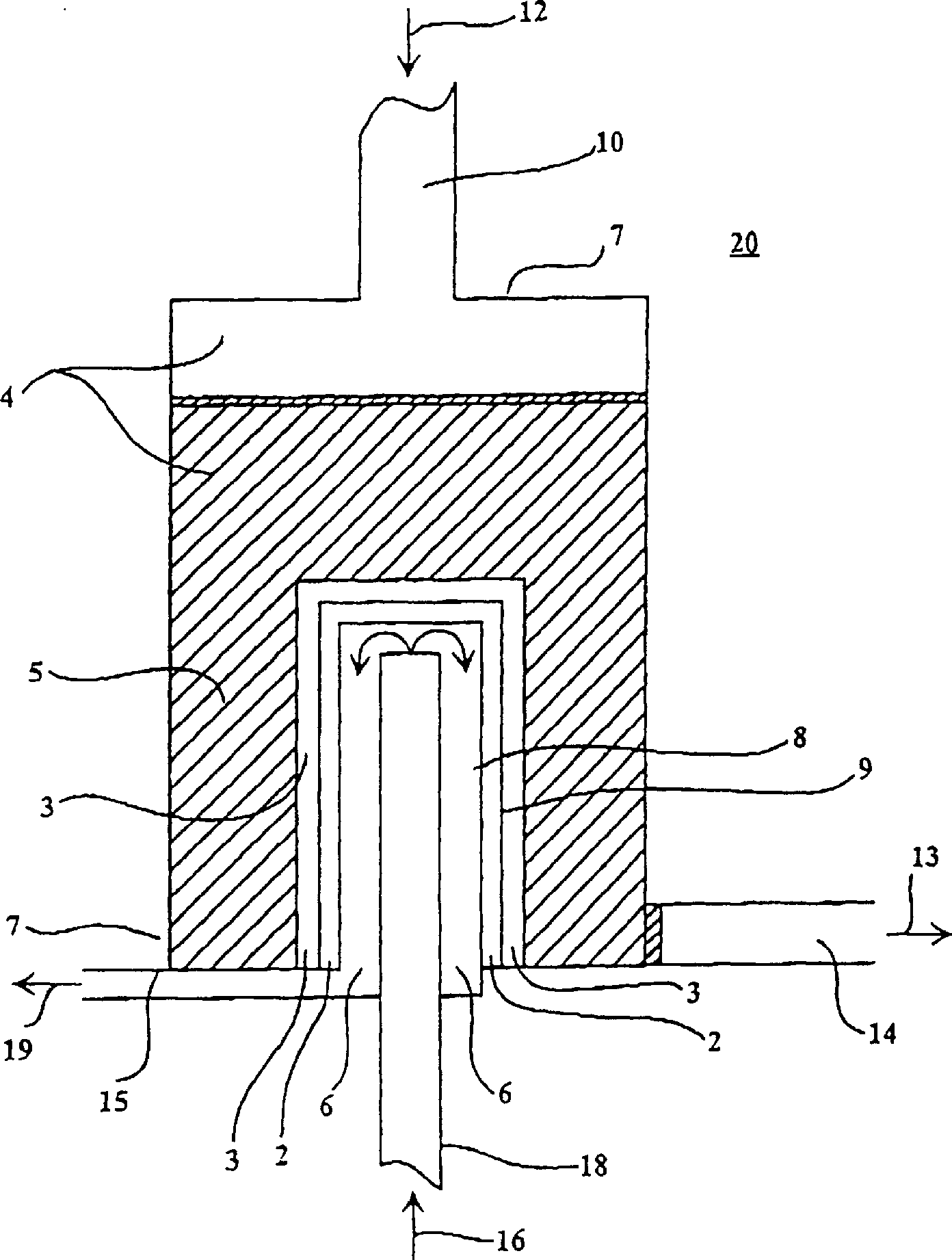
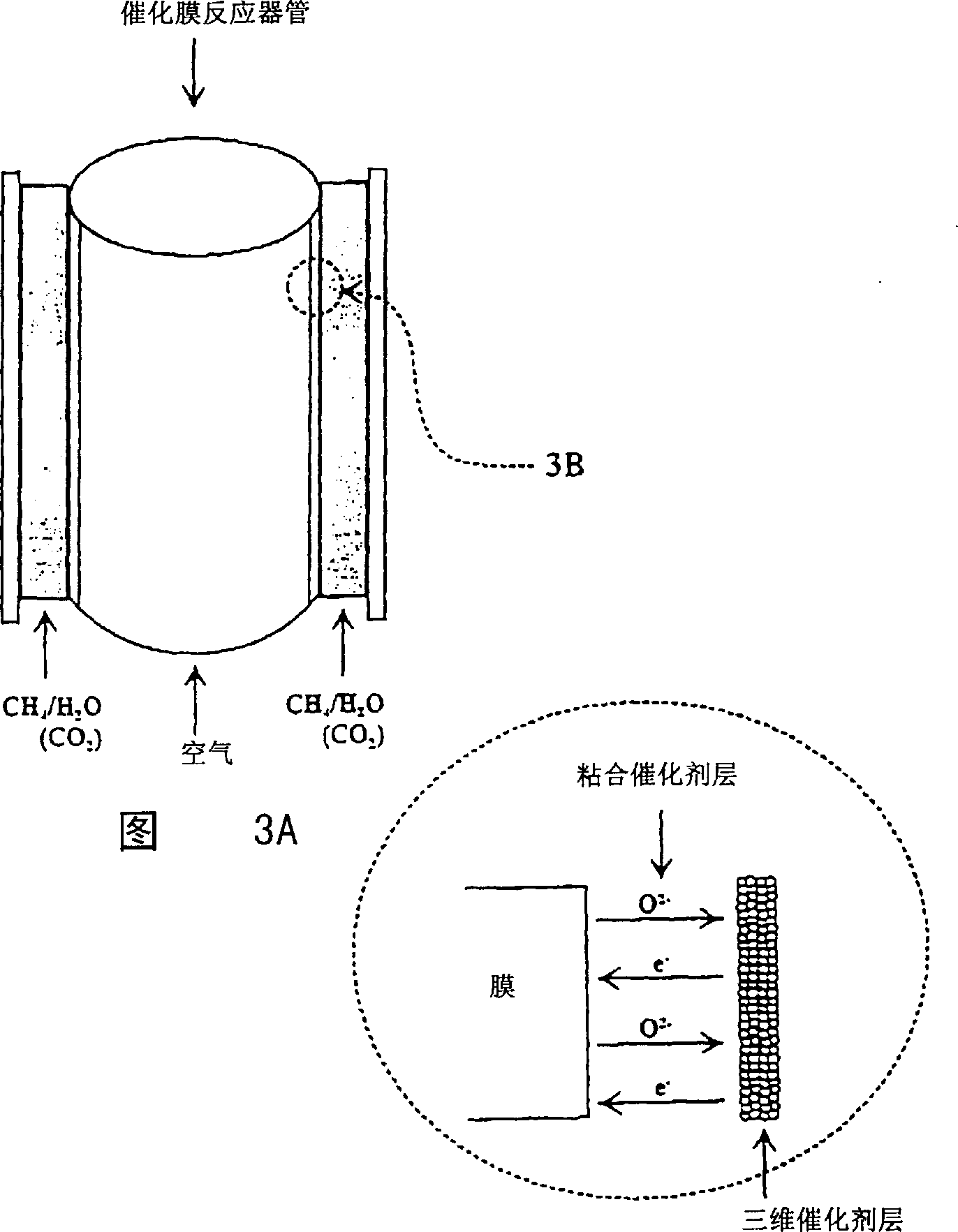
Catalytic membrane reactor with two component three dimensional catalysis
A catalytic membrane reactor, catalyst technology, applied in the reaction of gas and gas under the catalytic active body, chemical/physical/physicochemical fixed reactor, heterogeneous catalyst chemical elements, etc., can solve the problem of affecting reaction products, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Production of synthesis gas in a reactor with and without bonded catalyst layers.
[0112] By chemical formula Sr 1.7 La 0.3 Ga 0.6 Fe 1.4 o 5.15 A single-phase material for the fabrication of tubular membranes with one end closed. The powder of this composition is firstly prepared by conventional solid-state synthesis method, specifically as described in PCT / US96 / 14841 and Example 5. This single-phase powder was pressed into a tube shape by isostatic pressing method, followed by sintering to obtain a dense and strong tubular membrane. The grinding and sintering steps can be repeated, if desired, to ensure that the material is a single phase prior to isostatic pressing to form the tube.
[0113] Coating a layer of La on the inner surface (reducing surface) of the tubular membrane 0.8 Sr 0.2 CoO 3 (LSC) used as an oxidation / reduction catalyst; or coated with a metal on the oxidation / reduction catalyst, such as 5% by weight Pd on LSC.
[0114] The ou...
Embodiment 2
[0119] Example 2: Syngas Production in Reactors with and without Packed Bed Catalysts
[0120] Same as embodiment 1, by chemical formula Sr 1.7 La 0.3 Ga 0.6 Fe 1.4 o 5.15 A single-phase material for the fabrication of tubular membranes with one end closed.
[0121] A layer of La was coated on the inner surface of the tubular membrane 0.8 Sr 0.2 COO 3 (LSC) was used as a redox catalyst. Adhesive catalyst coated on the outer surface of the tubular membrane: at La 0.8 Sr 0.2 MnO 3 Ni on (20% by weight).
[0122] exist figure 1 The synthesis gas reactor shown compares tubular membranes. One reactor is supplied with Al in its oxidation zone 2 o 3 A packed bed of granules that were previously coated with La 0.8 Sr 0.2 MnO 3 Powder with Ni (10% by weight) on top: while one reactor had no packed bed. In both cases, air was used as the oxygen-containing gas passing through the interior of the tubular membranes and an 80% by volume methane mixture in helium was used ...
Embodiment 3
[0126] Example 3: Syngas production in reactors with different bonded catalyst layers
[0127] As described in Example 1, by chemical formula Sr 1.7 La 0.3 Ga 0.6 Fe 1.4 o 5.15 A single-phase material for the fabrication of tubular membranes with one end closed.
[0128] The inner surface of the tubular membrane is coated with a layer of La 0.8 Sr 0.2 COO 3 Used as a redox catalyst.
[0129] The outer surface of a tubular membrane is coated with La 0.8 Sr 0.2 MnO 3 . The outer surface of another tubular membrane was coated with La with Ni (20% by weight) on it. 0.8 Sr 0.2 MnO 3 . These two catalysts were used as the bonded catalyst layers on the oxidized surfaces of the two membranes, respectively.
[0130] in such as figure 1 The synthesis gas reactor shown compares tube membranes with different bonded catalyst layers. In both cases, the reactor was supplied with Al coated with Ni (5% by weight) in its oxidation zone. 2 o 3 Particle packed bed. Both used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com