Pharmaceutical composition with sustained release function and its preparing process
A technology of composition and medicine, which is applied in the field of pharmaceutical composition and its preparation, and can solve problems such as skin itching, rash, and affecting the clinical application of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
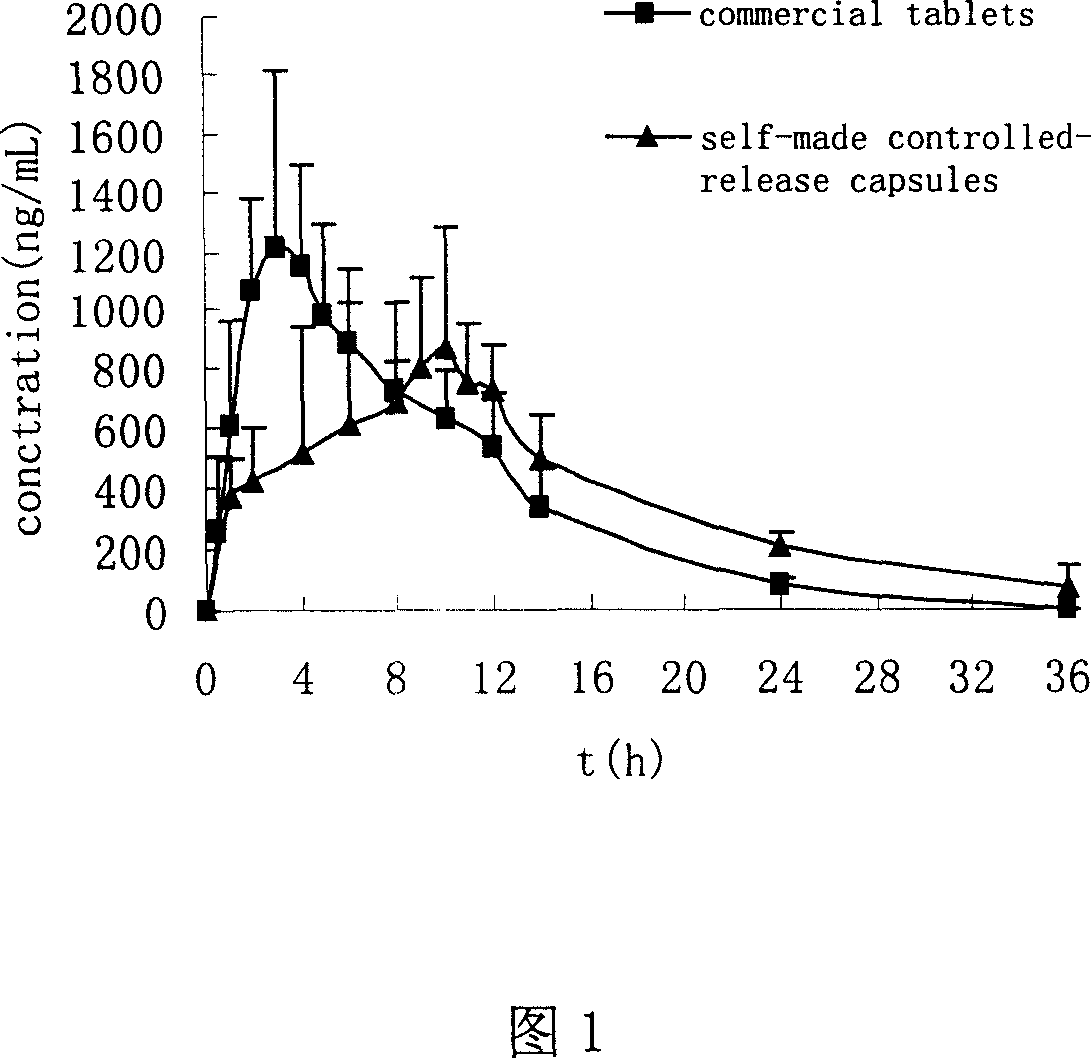
experiment example 1
[0031] 1. Experimental method:
[0032] Six healthy Beagle dogs, body weight (20±3kg), were fasted for 12 hours before taking the medicine, and the interval between two medicines was 7 days. According to the design of randomized crossover experiment, six Beagle dogs were orally administered Zhengqing Fengtongning Controlled-release Capsules (A) and commercially available Zhengqing Fengtongning Sustained-release Tablets (B) respectively, with a dose of 120 mg. Oral administration after fasting for 12 hours. The blood collection time points are: (unit: h)
[0033] Controlled release capsules: 0, 1, 2, 4, 6, 8, 9, 10, 11, 12, 14, 24, 36
[0034] Sustained-release tablets: 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 24
[0035] About 2 mL of blood was collected from the foreleg vein, placed in a heparin-coated centrifuge tube, and immediately centrifuged at 3,500 rpm for 10 minutes to absorb the upper layer of plasma, and stored in a -20°C refrigerator until analysis.
[0036] (1...
experiment example 2
[0051] Experimental Example 2 Prescription Screening Experiment
[0052] Get 300g drug-containing pill cores respectively, and carry out the research on the following coating solution prescriptions:
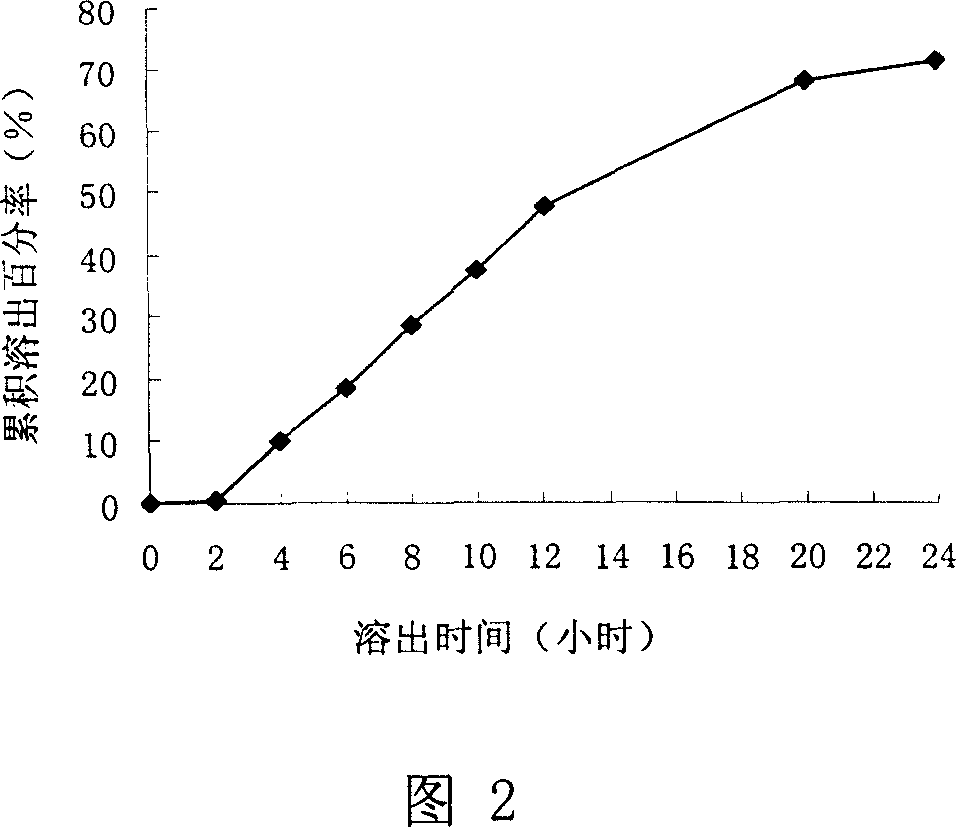
[0053] Prescription one:
[0054] Eudragit NE 30D 75g Sodium Lauryl Sulfate 0.2g
[0055] Talc powder 9.24g add water to 250mL
[0056] Result: The dissolution rate of the capsules was investigated, and the results are shown in Figure 2.
[0057] As can be seen from accompanying drawing 2, the dissolution rate is slower, and the cumulative dissolution percentage in 24 hours is less than 75%. The amount of Eudragit NE 30D should be reduced.
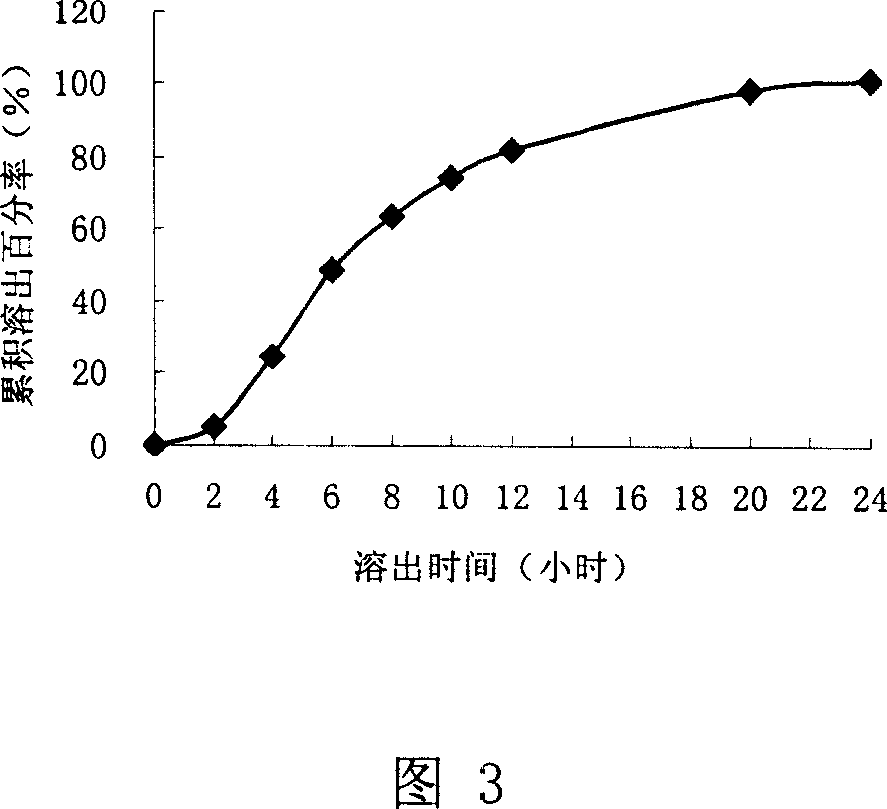
[0058] Prescription two:
[0059] Eudragit NE 30D 65g Sodium Lauryl Sulfate 0.3g
[0060] Talc powder 9.24g add water to 250mL
[0061] Result: The dissolution rate of the capsules was investigated, and the results are shown in Figure 3
[0062] It can be seen from accompanying drawing 3 that the dissolution rate is faster, and the cumu...
Embodiment 1
[0084] Microcrystalline Cellulose 172g Sinomenine Hydrochloride 120g
[0085] Hypromellose 3.43g Acrylic resin (Eudragit NE 30D) 58.67g
[0086] Sodium Lauryl Sulfate 0.18g Talc 8.34g
[0087] As above raw materials, put 32-24 mesh microcrystalline cellulose blank cores in a centrifugal coating granulator, use sinomenine hydrochloride (below 100 mesh) as powder, and use 2% hydroxypropyl methylcellulose aqueous solution as the binder According to the following parameters, start the centrifugal coating granulator: main engine speed 200rpm, blast flow rate 15L / min, jet flow rate 10-20L / min, jet pressure 0.025-0.1MPa, spray pump speed 5-25r / min, The powder feeding speed is 5~10g / min. Until the powder is all layered on the mother core. Dry at room temperature to obtain a pill core. Add sodium lauryl sulfate and talcum powder into water and stir for 2 hours to make it uniform. Slowly pour the above suspension into Eudragit NE 30D and stir evenly. Filter through an 80-mesh sieve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com