Method for making aromatic carbonates
A technology of diaryl carbonate and aromatic hydroxy, which is applied in the field of carbonylation of aromatic hydroxy compounds, and can solve problems such as low commercial purpose and low reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
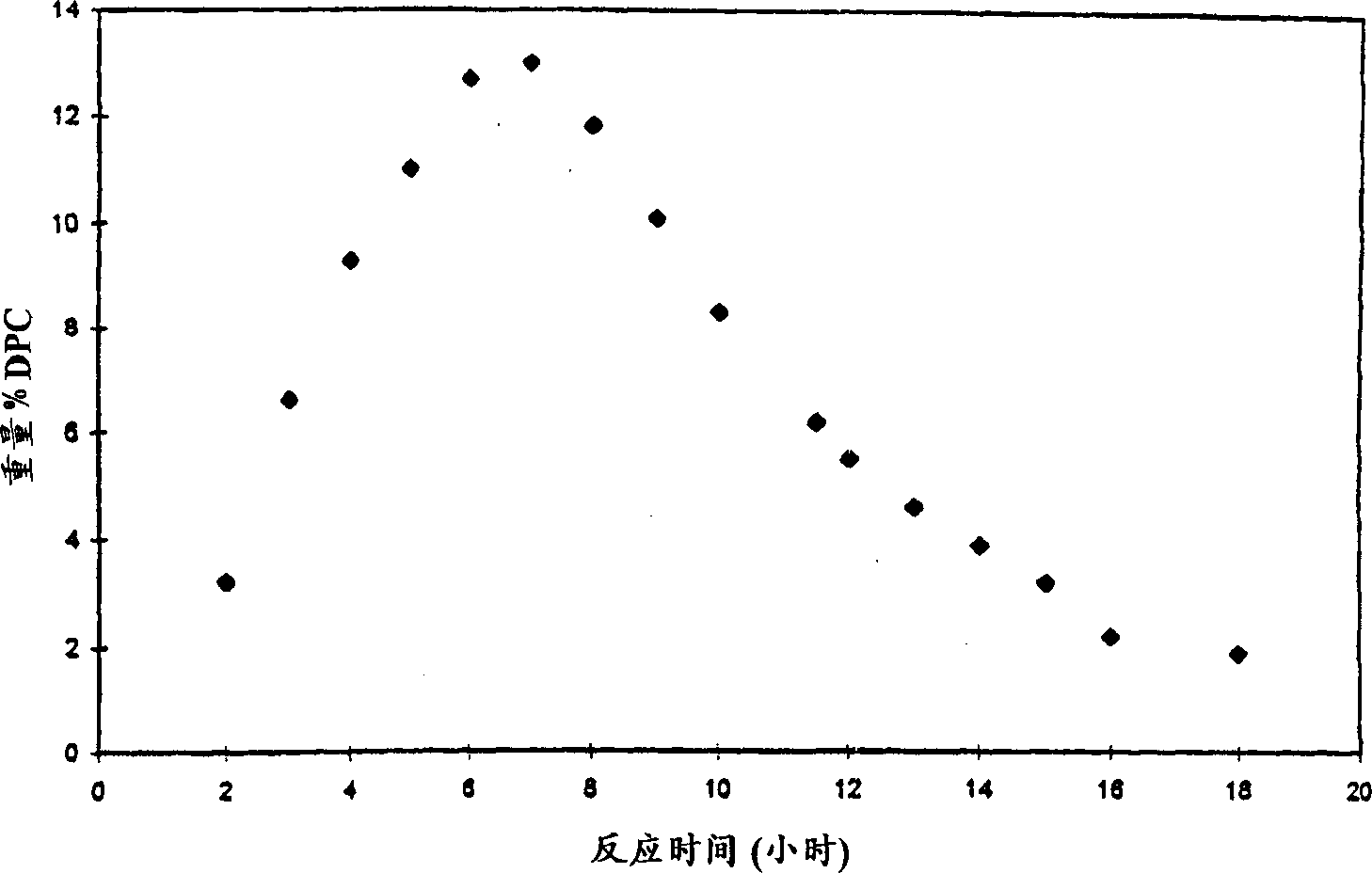
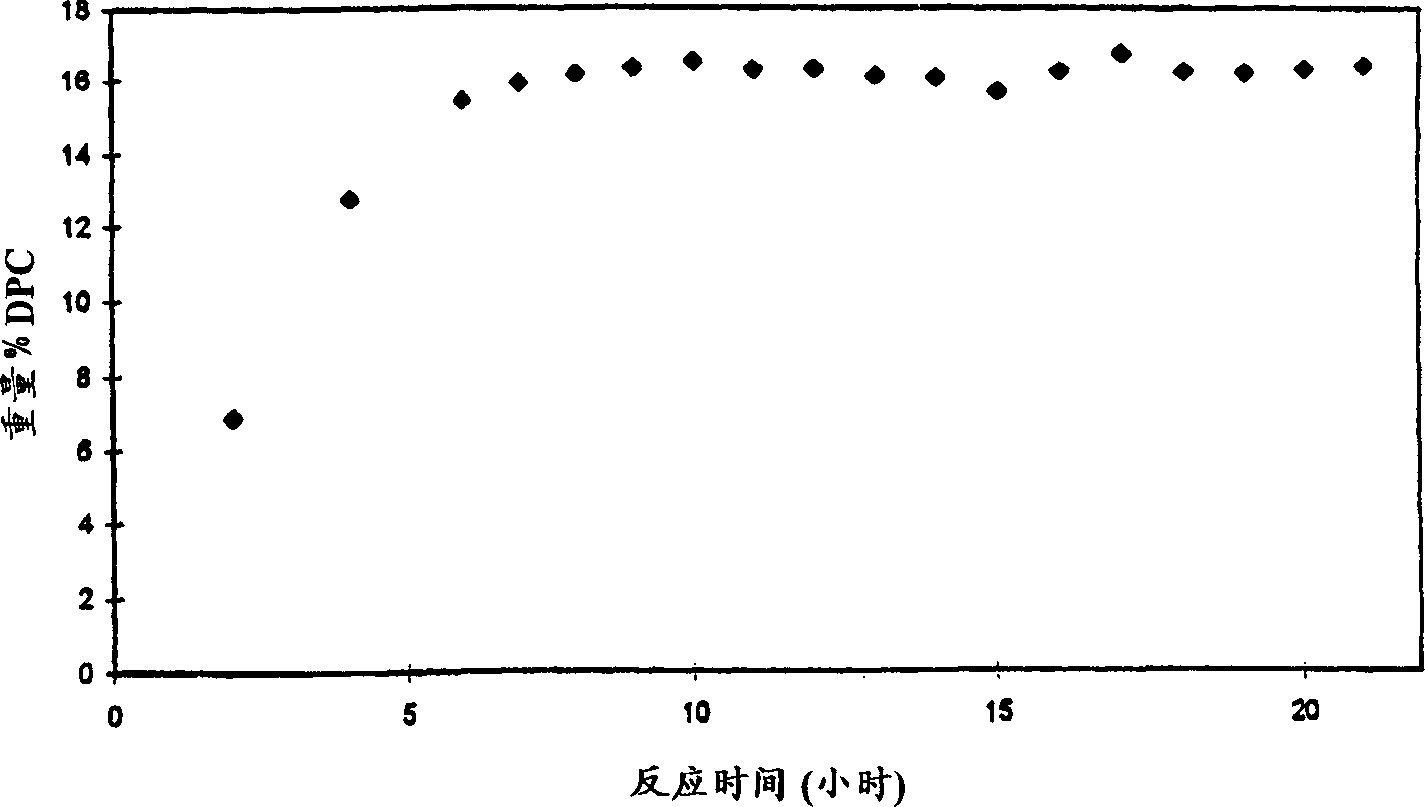
[0028] As disclosed in the aforementioned U.S. Patent No. 5,399,734, in a reactor system with a certain gas flow composition, 58.8837 grams of phenol (626 mmoles), 0.0055 grams of Pd(acac) were added 2 (0.018 mmol), 0.2067 g of PbO (0.927 mmol), 3.1982 g of hexaethylguanidinium bromide (10.4 mmol) and 38 g of molecular sieves (activated by drying) to produce 52 equivalents of lead per equivalent of palladium and 52 equivalents per equivalent of palladium Palladium 578 Equivalent Bromide Source Reaction Composition. The reactor was closed and heated to 100°C with stirring and a mixture of 9.1% oxygen in carbon monoxide was fed at a flow rate of 330 ml / min at a pressure of about 1320 psig. The gas flow was continued for 1.5 hours, after which a sample of the reaction mixture was taken for HPLC analysis. The yield of diphenyl carbonate was 16.6 g (27% by weight). The reaction rate for 1.5 hours was 0.84 moles of diphenyl carbonate per liter per hour. The palladium conversion n...
Embodiment 2
[0030] Embodiment 2 (comparison)
[0031] In the reactor system with a certain gas flow composition, add 60.9546 grams of phenol (648 mmol), 0.0049 grams of Pd(acac) 2 (0.016 mmol), 0.2111 gram of PbO (0.947 mmol), 3.2111 gram of hexaethylguanidinium bromide (10.4 mmol) and without adding any desiccant, to obtain 59 equivalents of lead per equivalent of palladium and 650 equivalents of bromine per equivalent of palladium The reaction composition of the compound source. The reactor was closed and heated to 100°C with stirring, and a mixture of 9.1% oxygen in carbon monoxide was fed at a flow rate of 330 ml / min at a pressure of 1320 psig. The gas flow was continued for 1.5 hours, after which a sample of the reaction mixture was taken for HPLC analysis. The yield of diphenyl carbonate was 14.8 g (23% by weight). The 1.5 hour reaction rate was 0.72 moles of diphenyl carbonate per liter per hour. The conversion number of palladium was 4302. The bromide promoter had a conversio...
Embodiment 3
[0034] In a reactor with a certain gas flow composition, add 59.5839 grams of phenol (633 mmol), 0.0051 grams of Pd(acac) 2 (0.017 mmol), 201.2 g of PbO (0.902 mmol), 3.2062 g of hexaethylguanidinium bromide (10.4 mmol), resulting in a reaction composition containing 53.8 equivalents of lead per equivalent of palladium and 612 equivalents of bromide source per equivalent of palladium . The reactor was closed and heated to 100°C with stirring, and a mixture of 9.1% oxygen in carbon monoxide was fed at a flow rate of 330 ml / min at a pressure of 1320 psig. The gas flow was continued for 1.5 hours, after which a sample of the reaction mixture was taken for HPLC analysis. The yield of diphenyl carbonate was 14.9 g (23.7% by weight). The 1.5 hour reaction rate was 0.74 moles of diphenyl carbonate per liter per hour. The conversion number of palladium was 4174. The bromide promoter had a conversion number of 6.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com