Triphenyl diacetylene compound with reaction and liquid crystal polymer containing the compound
A technology of acetylene compounds and liquid crystal polymers, applied in the direction of liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of non-reactivity, inability to form films, and inability to polymerize polymers, etc., to achieve increased pitch and increase The effect of large reflection width and high optical anisotropy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
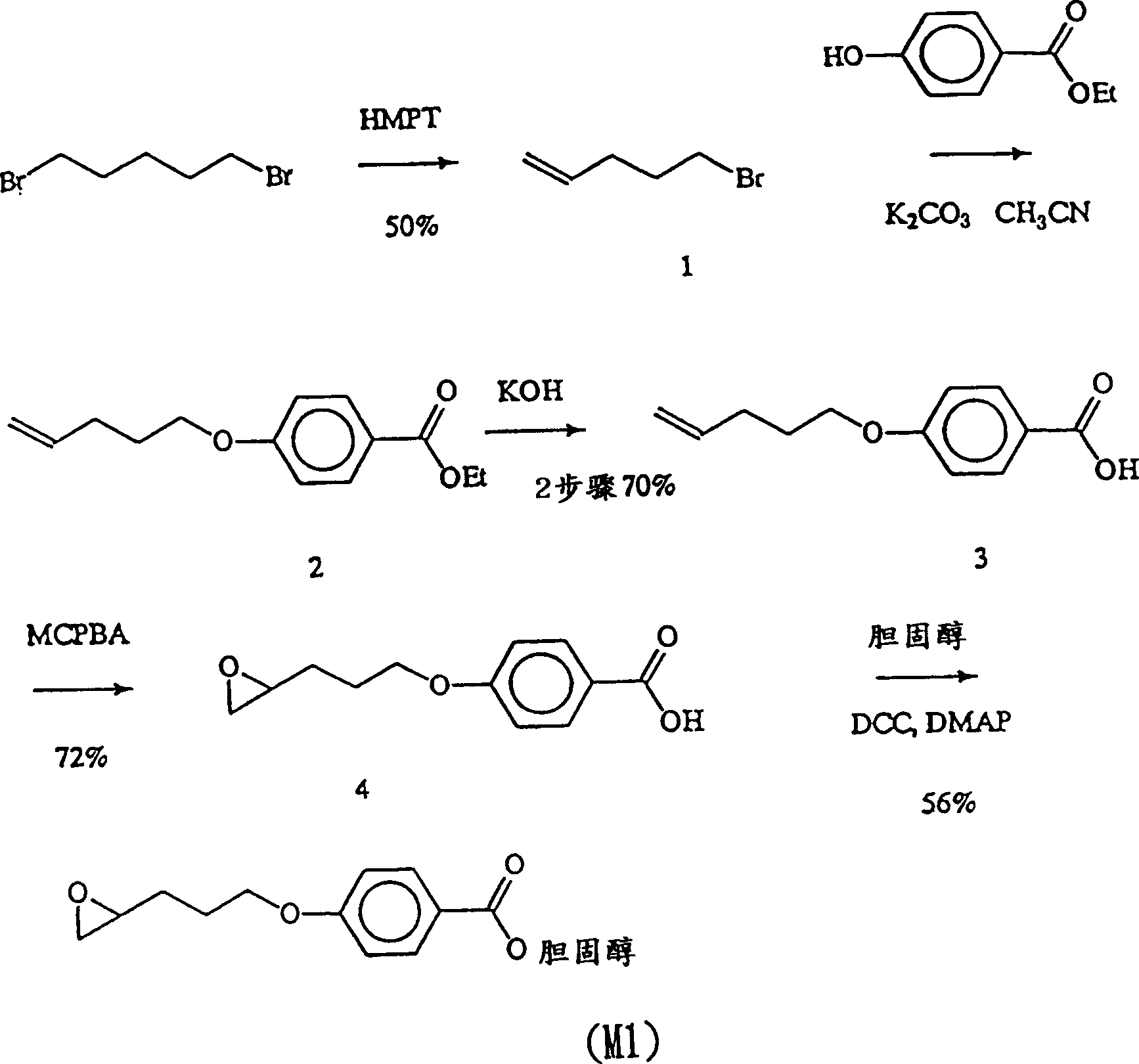
[0063] Example 1: Synthesis of monomer M1 cholesteryl 4-[3-(2-oxiranyl)propoxy]benzoate (cholesteryl 4-[3-(2-oxiranyl)propoxy]benzoate)
[0064] For synthesis steps, please refer to figure 1 .
[0065] Step 1: Synthesis of 5-bromopentene (1)
[0066] Put 100g (0.41mol) of 1,5-dibromopentane (1,5-dibromopentane) in a 200ml double-neck bottle and install a liquid feeding tube and a distillation tube respectively. 50 g of HMPT (hexamethyl phosphoroustriamide) (0.28 mol) were added. After heating the double-neck flask to 180°C, seal the system, and add HMPT dropwise, cool the round-bottom flask with liquid nitrogen, react until no solution evaporates, take out the collected solution, purify it with silica gel column chromatography, wash The dehydration is n-hexane. Yield 50%.
[0067] Step 2: Synthesis of 4-(4-pentenyloxy)benzoic acid (4-(4-pentenyloxy)benzoic acid) (3)
[0068] Mix 15g (0.1mol) of 5-bromopentene (1) with 20g (0.12mol) of ethyl4-hydroxybenzoate (ethyl4-hydr...
Embodiment 2
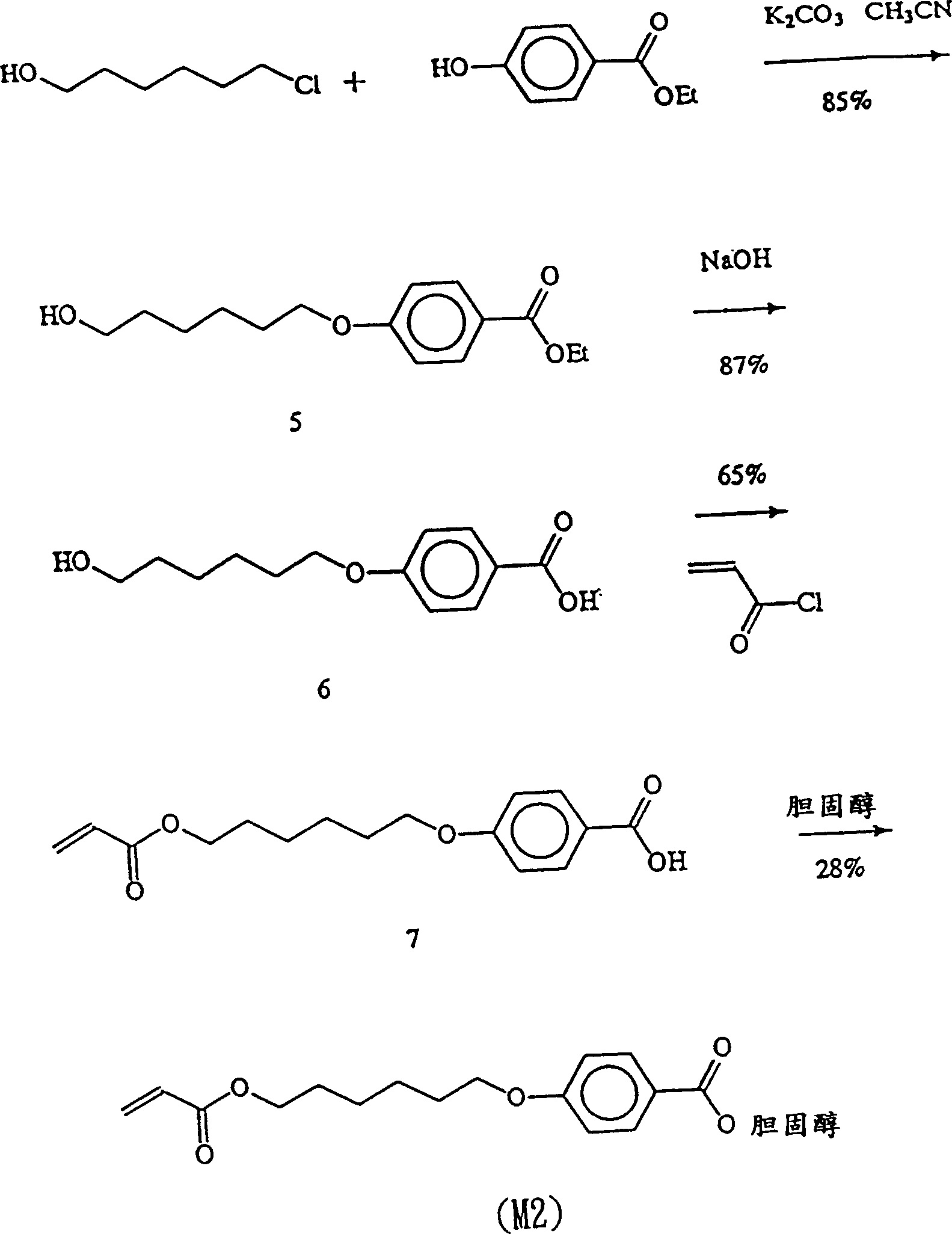
[0073] Example 2: Synthesis of monomer M2 cholesteryl [4-6-acryloyloxyhexoxy] benzoate (cholesteryl-[4-(6-acryloyloxyhexoxy)]benzoate.
[0074] Step 1: Synthesis of ethyl-4-(6-hydroxyhexyl-1-oxy)oxy)benzoate (ethyl-4-(6-hydroxyhexyl-1-oxy)benzoate) (5)
[0075] For synthesis steps, please refer to figure 2 .
[0076] 8.2 g (60 mmol) of 6-chlorohexanol, 5.0 g (30 mmol) of ethyl 4-hydroxybenzoate and 12.4 g (90 mmol) of potassium carbonate were dissolved in 150 ml of acetonitrile, and heated to reflux for 24 hours. Concentrate to remove acetonitrile, dissolve in acetic acid, wash the organic layer three times, dry and concentrate, and purify by silica gel column chromatography, the eluent is ethyl acetate and n-hexane (1:4, v / v). A colorless liquid was obtained. Yield 85%.
[0077] Step 2: Synthesis of 4-(6-hydroxyhexyl-1-oxy)benzoic acid (4-(6-hydroxyhexyl-1-oxy)benzoic acid)(6)
[0078] Put 8.00g (30mmol) of compound (5) and 1.4g (35mmol) of NaOH in a 500ml round bottom ...
Embodiment 3
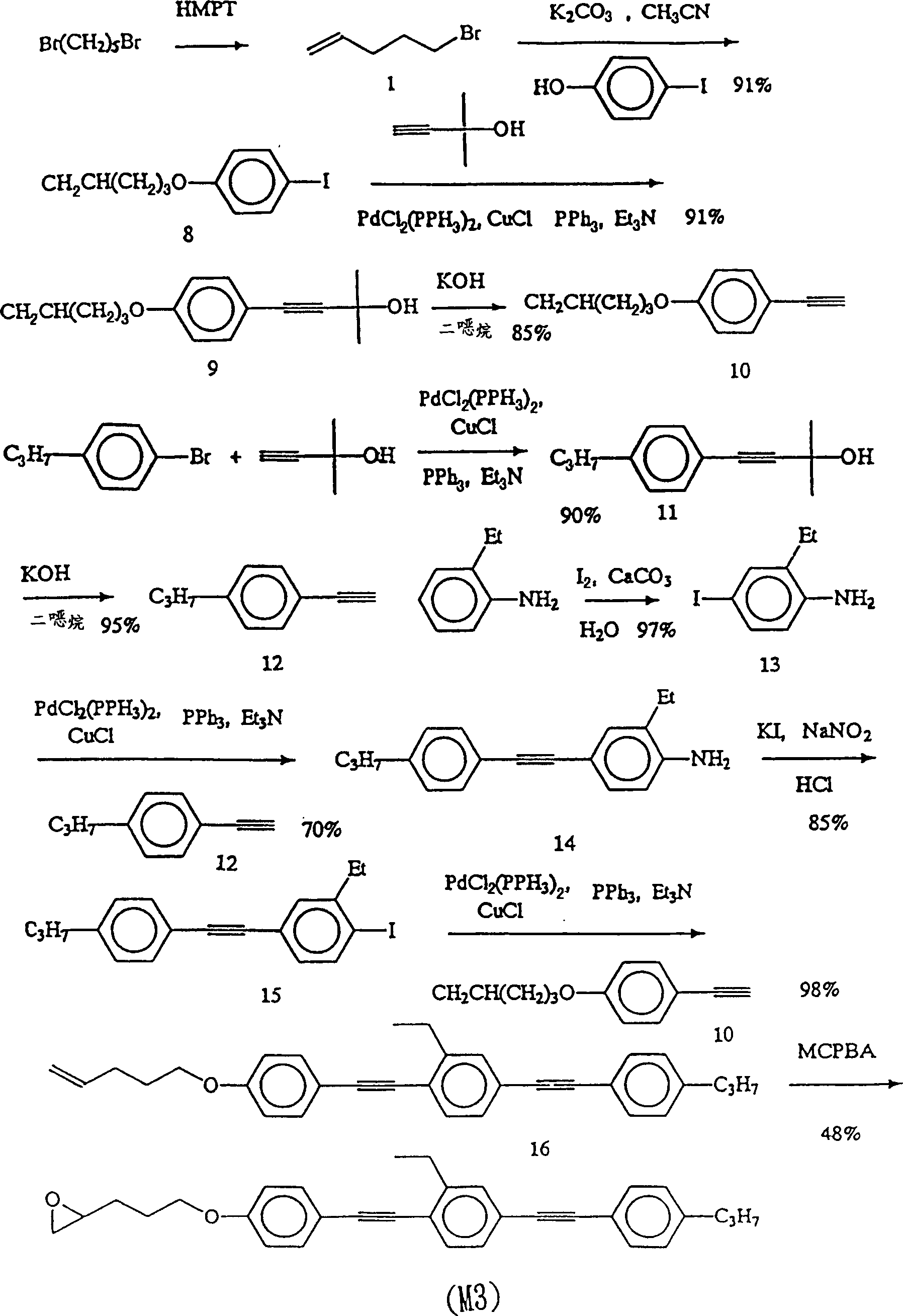
[0083] Example 3: Synthesis of monomer M3 2-3-[4-(2-2-ethyl-4-[2-(4-propylphenyl)-1-ethynyl]phenyl-1-ethyl) Phenoxy]propyl oxirane (2-3-[4-(2-2-ethyl-4-[2-(4-propylphenyl)1-ethynyl]phenyl-1-ethyl)phenoxy]propyl oxirane).
[0084] For synthesis steps, please refer to image 3 .
[0085] Step 1: Synthesis of 1-iodo-4-(4-pentenyloxy)benzene (1-iodo-4-(4-pentenyloxy)benzene) (8)
[0086] Put 18g (0.12mol) of 5-bromopentene (1), 22g (0.1mol) of iodophenol, 27.6g (0.2mol) of potassium carbonate and 0.5g (3mmol) of potassium iodide in a round bottom flask, add acetonitrile (800ml) was dissolved and heated to reflux for 24 hours. Concentrate to remove acetonitrile, dissolve with ether, wash the organic layer three times with water, dry and concentrate, and purify by silica gel column chromatography, the eluent is ethyl acetate and n-hexane 1:4. A white solid was obtained. Yield 91%.
[0087] Step 2: Synthesis of 2-methyl-4-[4-pentenyloxy]phenyl]-3-butyn-2-ol (2-methyl-4-[4-(4-pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com