Cloning, Expression and bioactivity of new actinion toxin
An amino acid and technology in the sequence table, applied in the field of protein structure, can solve the problems of small detoxification, difficulty in collecting venom, and low production of sea anemones, etc., and achieve enhanced atrial contraction, high application prospects and industrial development value, strong The effect of promoting contraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example One The extraction and RT-PCR amplification of the tentacle total RNA
[0046] Extraction of total RNA and synthesis of cDNA: Isolate the tentacles of a sea anemone lateralis, extract the total RNA of the tentacles by guanidine isothiocyanate method, and remove the protein by phenol / chloroform extraction. Obtain 100 μg of total RNA from the venomous gland, and its A 260 / A 280 = 2.03, two clear bands of 18s and 28s can be seen through 1% formaldehyde denaturing gel electrophoresis, the ratio> 1, and several specific RNA bands (see figure 1 ), indicating that the integrity of total RNA was good. 5 μg of RNA was reverse-transcribed with Oligo-dT to synthesize the first-strand cDNA, and 20 μl of the first-strand cDNA product was obtained.
[0047] Primer design and RT-PCR amplification were based on: According to the amino acid sequences at both ends of Ap-B, oligo primer design software was used to assist analysis, and two degenerate primers were designed. Amin...
Embodiment 2
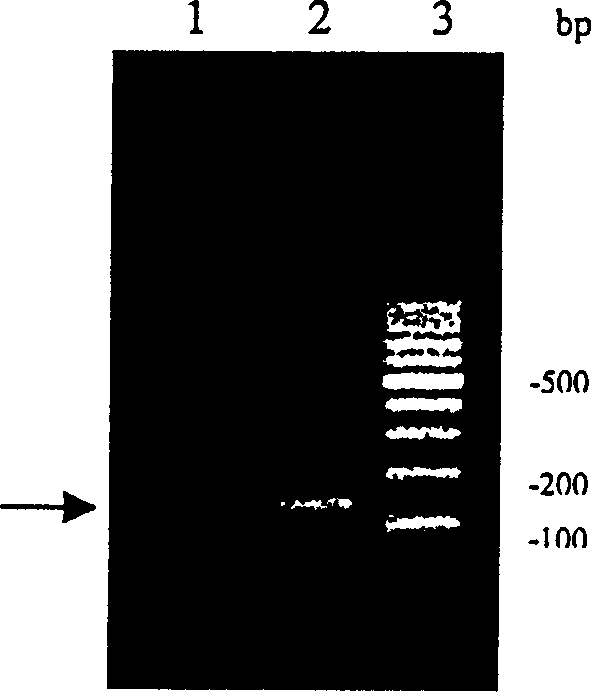
[0049] Add 1 μl cDNA single strand to every 50 μl PCR reaction volume as a template, perform PCR amplification, and detect by electrophoresis. It is found that the expected specific amplification band appears around 150 bp (see figure 2 ), recycle the tape. Example 2 Determination and Analysis of Recombinant Sea Anemone Neurotoxin Gene Sequence
[0050] The recovered electrophoresis products were connected to the T vector, transformed into DH5α Escherichia coli, and the recombinant clones were selected for sequencing. A total of 17 clones were determined, and Blast homology analysis showed that 8 of them are neurotoxin gene sequences, and the length of these 8 toxin genes is 141bp, encoding a toxin protein with a length of 47 amino acids. The toxin protein number is Sea anemone toxin-su, whose amino acid sequence is not identical with the reported sea anemone neurotoxin protein, is a new toxin protein.
[0051] At present, there are more than 20 kinds of sea anemone neuroto...
Embodiment 3
[0059] In the PCR amplification reaction, there will be about 10-4 mismatches with ordinary Taq enzymes. Since the target fragment is small, the number of PCR cycles does not exceed 30, which reduces the probability of wrong inclusion. From the experimental results, This sequence appeared in multiple clones, indicating that the results of PCR have high reliability. Example 3 Construction of Recombinant Sea Anemone Neurotoxin Expression Plasmid
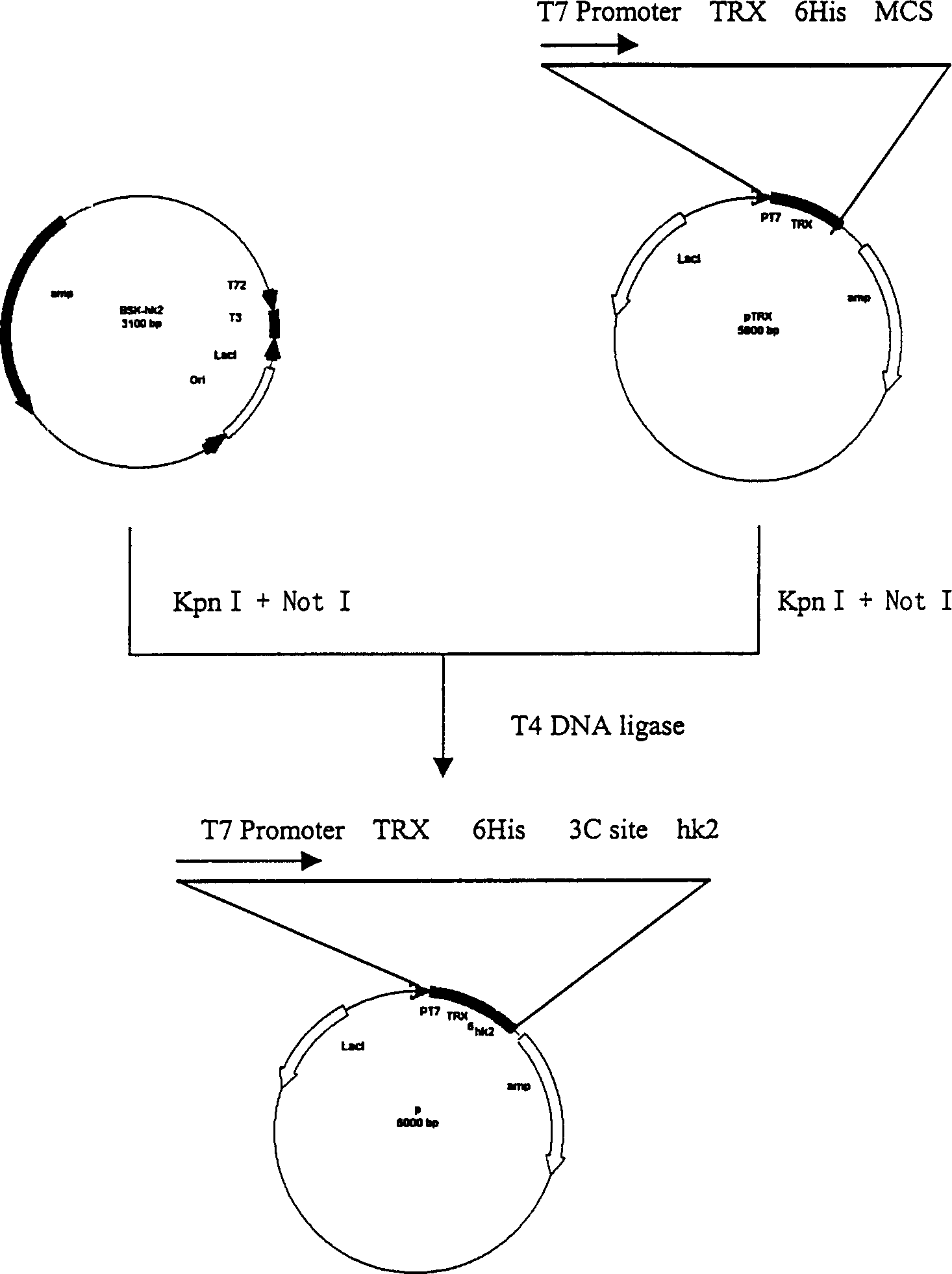
[0060] A pair of primers were synthesized according to the sequences at both ends of the su gene, the upstream primers contained Kpn I and Prescission Protease cleavage sites, and the downstream primers contained BamH I cleavage sites. Upstream primer (B1): 5'CGG GGT ACC CTG GAA GTT CTG TTC CAG GGG CCC GGG GTT
[0061] Kpn I Precision Protease site
[0062] CCG TGT TTG TGT GAC 3' downstream primer (B2): 5'CGC GGA TCC TTA TTA CTT CTT GCA GCA CCA GCC AAT G3’
[0063] Bam H I
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com