Utilization of ADAMTS-1 protein and method of screening aggrecanase activity regulator
A technology of aggrecan and protein, which is applied in the field of ADAMTS-1 protein use and aggrecanase activity regulator screening, which can solve the problems of low aggrecanase activity, abnormal cartilage remodeling, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
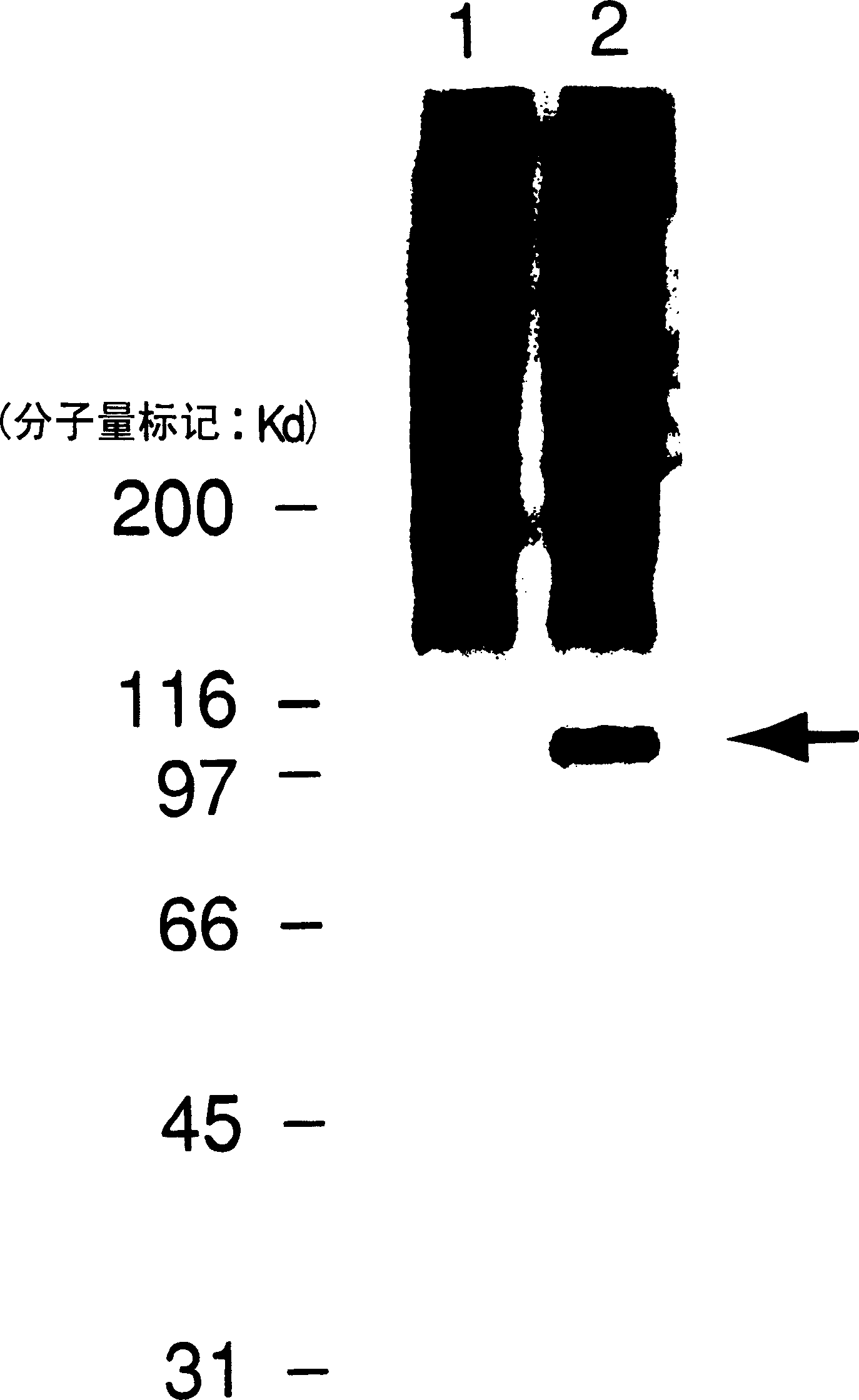
[0052] Hereinafter, the present invention will be specifically described based on examples, but the present invention is not limited to these. Example 1 (1) The recombinant human ADAMTS-1 protein was prepared using the base sequence shown in sequence number 1 of the sequence list: gtggcggccgcggctccaatg cagcgagctgtg and the base sequence shown in sequence number 2 of the sequence list: cactctagactccagccttct Reverse primers composed of tgctttccctg and human spleen cDNA library (Marath on-Ready cDNA; Clonetec Lab., Inc.; Palo Alto, California, U.S.). According to conventional methods, the human ADAMTS-1 was isolated by polymerase chain reaction (PCR) Protein gene (cDNA of approximately 3kbp). The base sequence of the obtained cDNA was analyzed, and the result was consistent with the base sequence of the human ADAMTS-1 gene described in JP 11-46781 A.
[0053] Next, after cutting the ends of the above cDNA with restriction endonuclease Xbal and restriction endonuclease Notl, agarose g...
preparation Embodiment 1
[0064] The recombinant ADAMTS-1 protein-containing component prepared in Example 1 (1) above was dissolved in physiological saline and the amount of ADAMTS-1 protein was 25 mg / mL, and then the obtained recombinant ADAMTS-1 protein solution was transferred to The ampoule tube is sealed to prepare the pharmaceutical composition of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com