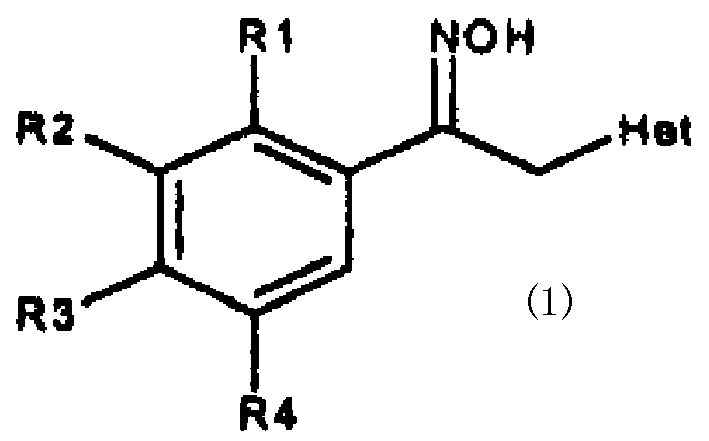
Substituted acetophenone oxime derivative and preparation process and use thereof
A technology of acetophenone oxime and its derivatives, which is applied in the application field of substituted acetophenone oxime derivatives and its preparation, and agricultural fungicides, which can solve the problems of improving agricultural cost and environmental impact, achieving good effect and less dosage , Improve the effect of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0037] 8.25g2-imidazolyl-3', 4'-dichloroacetophenone, 3.37g hydroxylamine hydrochloride, 2.5g sodium hydroxide and 40ml methanol were stirred at room temperature for 5 hours, then water was added to the reaction solution, and solids were precipitated. The solid was filtered off and dried to give 7.5 g of solid product. Yield: 76.99%. The physical and chemical constants are listed in Table 1. Example 3 Preparation of O-allyl-2-imidazolyl-2',4'-dichloroacetophenone oxime (compound 2)
Embodiment 3
[0038] 2.7g 2-imidazolyl-2', 4'-dichloroacetophenone oxime, 1.15g chloropropene, 0.6g potassium hydroxide and 50ml benzene were heated to reflux for 6 hours, then cooled to room temperature, the benzene layer was washed with water until neutral and After drying over anhydrous magnesium sulfate, the benzene was evaporated under reduced pressure, and the residue was separated through a chromatographic column equipped with silica gel (60-100 mesh) with acetone / petroleum ether (1:2) as the eluent. After separation and purification by column chromatography, 2.0 g of an oily product was obtained. Yield: 64.52%. The physical and chemical constants are listed in Table 1. Example 4 Preparation of O-allyl-2-imidazolyl-4'-trifluoromethylacetophenone oxime (compound 17)
Embodiment 4
[0039]1.09g2-imidazolyl-4'-trifluoromethylacetophenone oxime, 0.37g chloropropene, 0.24g sodium hydroxide and 30ml dichloromethane were stirred at room temperature for 20 hours, then the dichloromethane layer was washed with water until neutral It was dried over anhydrous magnesium sulfate, dichloromethane was distilled off under reduced pressure, and the residue was separated on a chromatographic column equipped with silica gel (60-100 mesh) with acetone / petroleum ether (1:2) as eluent. After separation and purification by column chromatography, 0.55 g of an oily product was obtained. Yield: 43.76%. The physical and chemical constants are listed in Table 1. Example 5 Preparation of O-methylenecyclopropyl-2-imidazolyl-2',4'-dichloro-5'-fluoroacetophenone oxime (compound 23)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com