A anti-enveloped virus recombinant polypeptide and its preparation method
An antiviral and viral envelope technology, applied in antiviral agents, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of expanding the application scope of colistin, achieve high targeting, reduce toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] [Example 1] Construction of a plasmid expressing an anti-hepatitis B virus polypeptide and preparation of a recombinant antiviral polypeptide
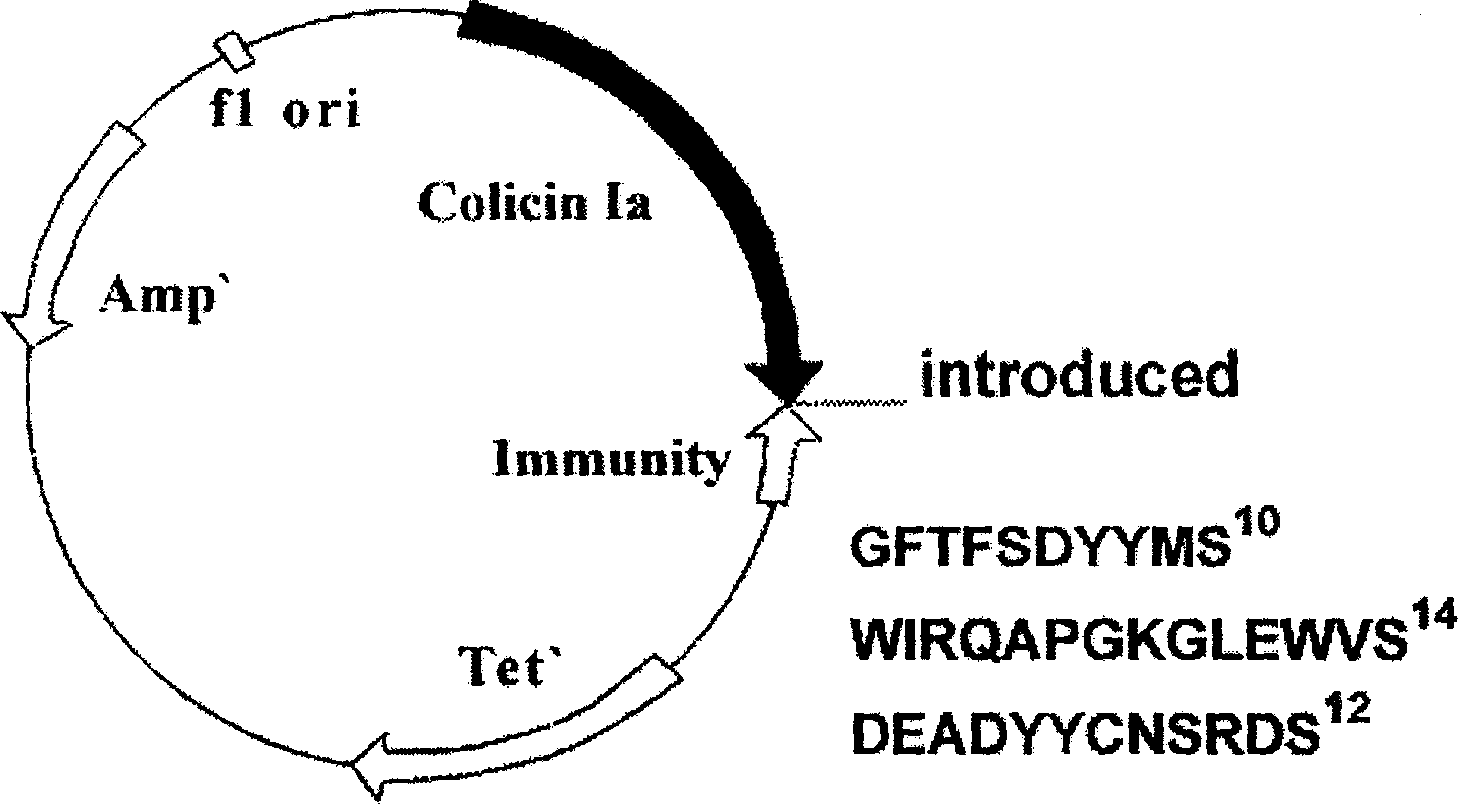
[0032] The original plasmid is pSELECT loaded with colistin Ia and immunity protein genes TM -1 commercial plasmid (plasmid size 8.3kb, Promega Company) (UCSF, donated by P.Gosh). Double-stranded oligonucleotide point mutation technology (QuickChange TM Kit, Strategeue Company) inserted the genes encoding the two peptide chains G28-V50 and E216-S228 in the anti-HBsAg single-chain antibody registered in GenBank AF236816 into the I626 site of the colicin Ia gene through 4-step point mutations, and prepared Mutant plasmids for antiviral polypeptides, (such as figure 1 shown). The mutant plasmid was transfected into E. coli TG1 engineering bacteria (AECOM, donated by K. Jakes) to prepare polypeptides.
[0033] The mutation program was carried out according to the Strategene QuikChange Site-Directed Mutagenesis Kit (Catalog#2005...
Embodiment 2
[0063]The genes of the G28-V50 and E216-S228 peptide chains in the anti-HBsAg scFv registered in GenBank AF236816 were connected to the carboxyl end of the colicin Ia channel domain (K544-I622) according to the method in Example 1, and prepared into an artificially combined For the plasmid of anti-HBV engineering polypeptide, transfect the mutated plasmid into engineering bacteria without plasmid, multiply the bacteria in large numbers (4L FB medium, 225rpm, 37°C; 6h), and centrifuge the bacterial cells (4°C, 6000g , 20min), take 4℃, 50mM boric acid buffer + 2mM EDTA+2mM DTT 50-80ml suspended bacteria, ultrasonically break (4℃, 40W, 1-2min), high-speed centrifuge to break the bacteria (4℃, 70000g, 1.5 h), take the supernatant and add streptomycin sulfate to precipitate DNA, dialyze overnight at 4°C in 50mM boric acid buffer + 2mM EDTA + 2mM DTT 2L, then load the sample on a CM ion exchange column, 0.1-0.3M NaCl + 50mM boric acid buffer After gradient elution, the anti-HBV engi...
Embodiment 3
[0065] According to the method of Example 1, the anti-HBV PreS1 scFv registered in GenBank AF427148 was connected to the amino terminal of the water-soluble pore structure domain of colistin Ia to form a recombinant plasmid, and obtain a recombinant polypeptide with a molecular weight of about 40,000;
[0066] The anti-HBV PreSl scFv registered in GenBank AF427148 was linked to the carboxyl terminus of the colicin Ia water-soluble pore structure domain to obtain a recombinant polypeptide with a molecular weight of about 40,000;
[0067] Link the anti-HBV PreSl scFv registered in GenBank AF427148 to the amino terminal of colicin Ia to obtain a recombinant polypeptide with a molecular weight of about 90,000;
[0068] The anti-HBV PreSl scFv registered in GenBank AF427148 was linked to the carboxyl terminus of colicin Ia to obtain a recombinant polypeptide with a molecular weight of about 90,000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com