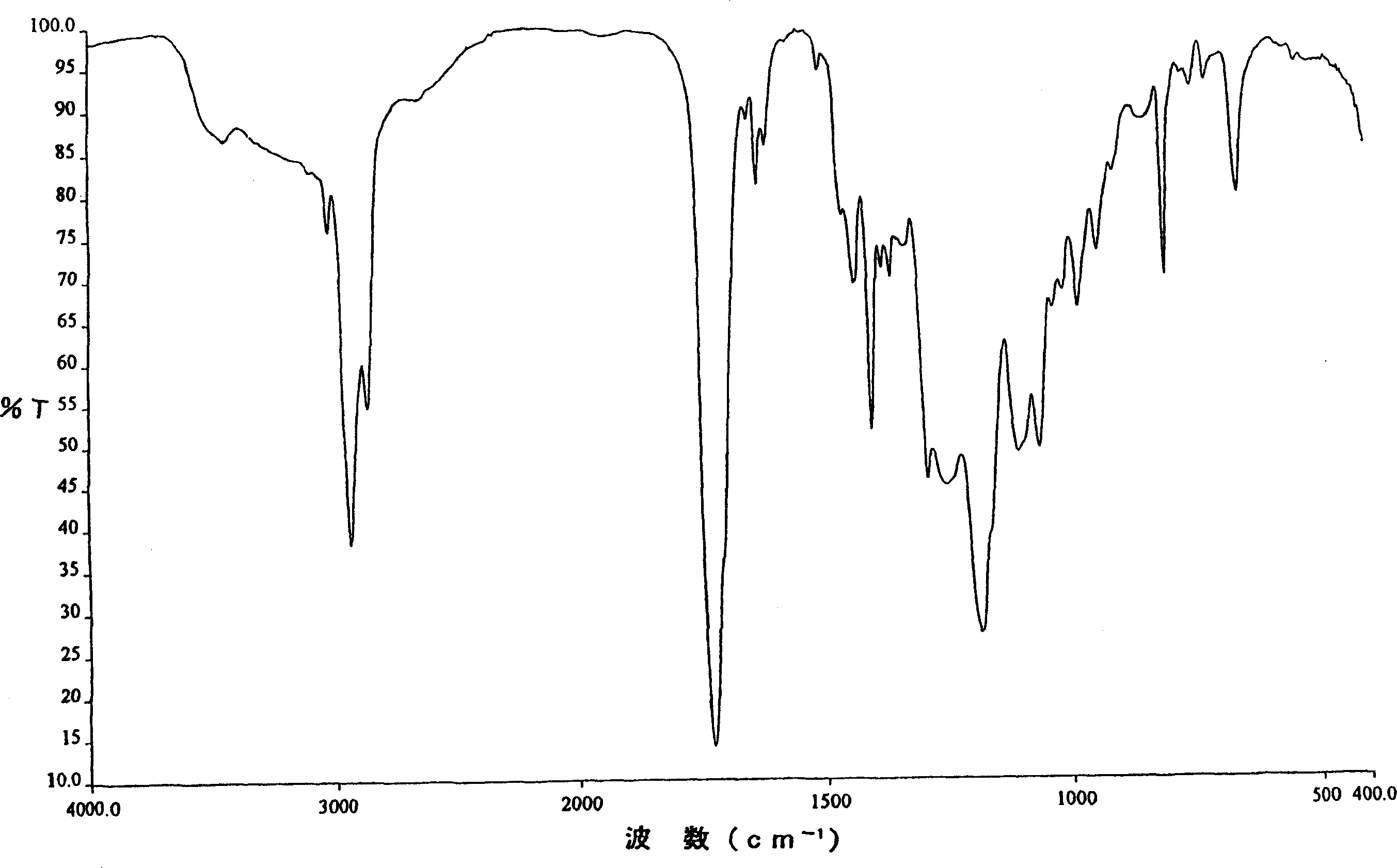
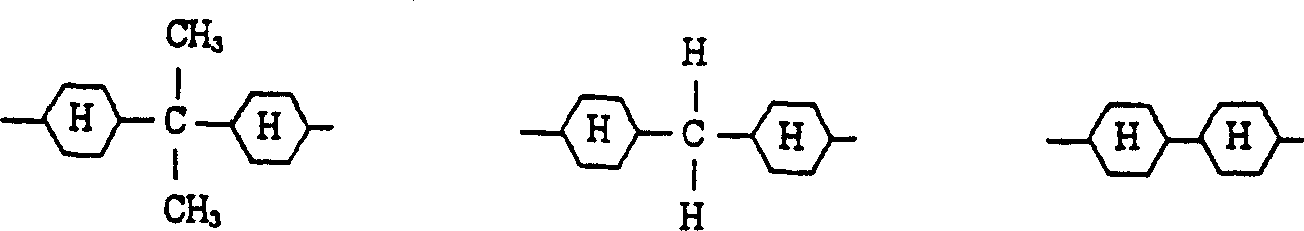
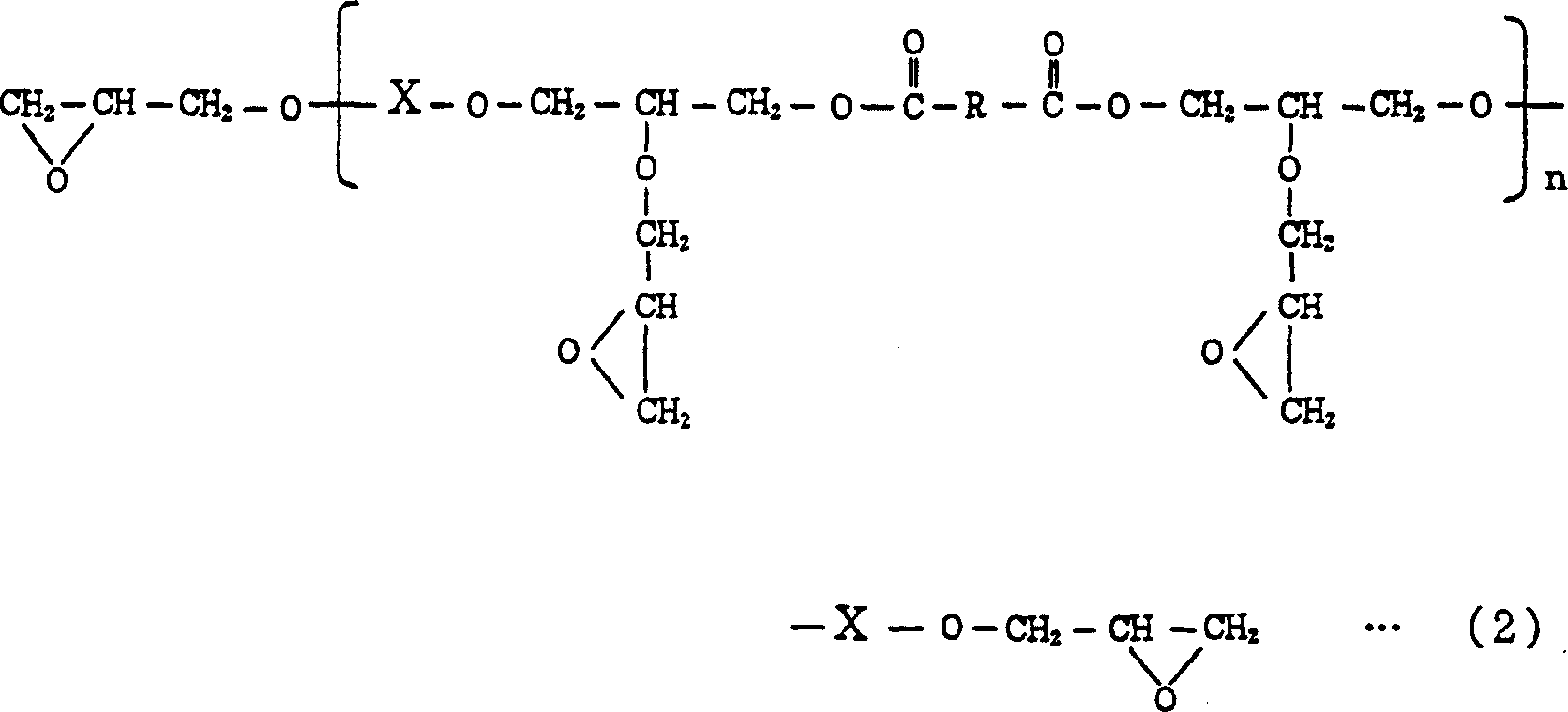Resin curable with actinic energy ray, photocurable/thermosetting resin composition containing the same
An active energy ray, resin composition technology, applied in optics, opto-mechanical equipment, photo-engraving process of patterned surface, etc., can solve the problems of moisture absorption resistance, poor long-term reliability, etc., and achieve excellent photocurability, high The effect of balancing flexibility and toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0098] Hereinafter, the present invention will be specifically described by way of examples and comparative examples. Of course, the present invention is not limited to the following examples. The following "parts" and "%" are subject to mass unless otherwise stated.
Synthetic example 1
[0100] In the flask equipped with gas inlet pipe, stirring device, cooling pipe and thermometer, put 172 parts of 1,4-cyclohexenedioic acid and hydrogenated bisphenol A diglycidyl ether (Nippon Cyclohexene) of 176 grams / equivalent Oxygen resin company make, YL-6663) 880 parts, it stirred at 100 degreeC under nitrogen atmosphere. After that, 0.65 parts of triphenylphosphine was added, and the temperature in the flask was increased to 150° C., while maintaining at 150° C. for about 90 minutes to obtain an epoxy compound (A′) having an epoxy equivalent of 438 g / equivalent.
[0101] Next, the temperature in the flask was cooled to below 70° C., 780 parts of epichlorohydrin and 635 parts of dimethyl sulfoxide were added, and the temperature was raised to 70° C. while stirring and maintained. Thereafter, 150 parts of 96% sodium hydroxide were intermittently added for 90 minutes, and reacted for an additional 3 hours. After the reaction is terminated, distill most of the excess epic...
Embodiment 1 and comparative example 1、2
[0109] Using the varnishes obtained in Synthesis Example 1 and Comparative Synthesis Examples 1 and 2 above, the formulation components in Table 1 were kneaded with a three-roll machine to obtain photocurable and thermosetting resin compositions. Table 2 shows the characteristic values of each composition.
[0110] Composition (parts by mass)
[0111] characteristics
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy equivalent | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com