Formulations comprising entrapped active ingredients and uses thereof
A technology of active ingredients and formulas, applied in the field of formulas containing encapsulated active ingredients and their uses, which can solve the problems of low anti-exudation and anti-inflammatory activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Preparation of GlcNAc Encapsulated by Liposomes
[0122] 33 μmol DSPC and 33 μmol Chol (or other compositions, see Table 1 below) were dissolved in 3 ml of chloroform:methanol (95:5, v / v) solution in a 100 ml Erlenmeyer flask. The organic solvent was evaporated under vacuum (600 mm Hg) at room temperature. After removing the last visible traces of solvent, the vacuum was maintained for at least 30 minutes.
[0123] 2 ml of GlcNAc (containing a total of 10 μCi of labeled and 5.38 mg of unlabeled GlcNAc in a 0.85% sodium chloride solution, with a final unlabeled to labeled molar ratio of 10000 tol) was added to the flask. The flask was placed in a water bath at 60°C (see Table 1 for temperatures of other compositions) for 5 minutes, and the dry lipid was suspended by vigorous shaking at 2500 rpm for 1 minute. Liposomes were annealed at the same temperature for 45 minutes. The prepared liposomes were then dispensed into four 1.5-ml Eppendorf tubes. Liposomes were dried...
Embodiment 2
[0167] In vitro release of GlcNAc from liposomes
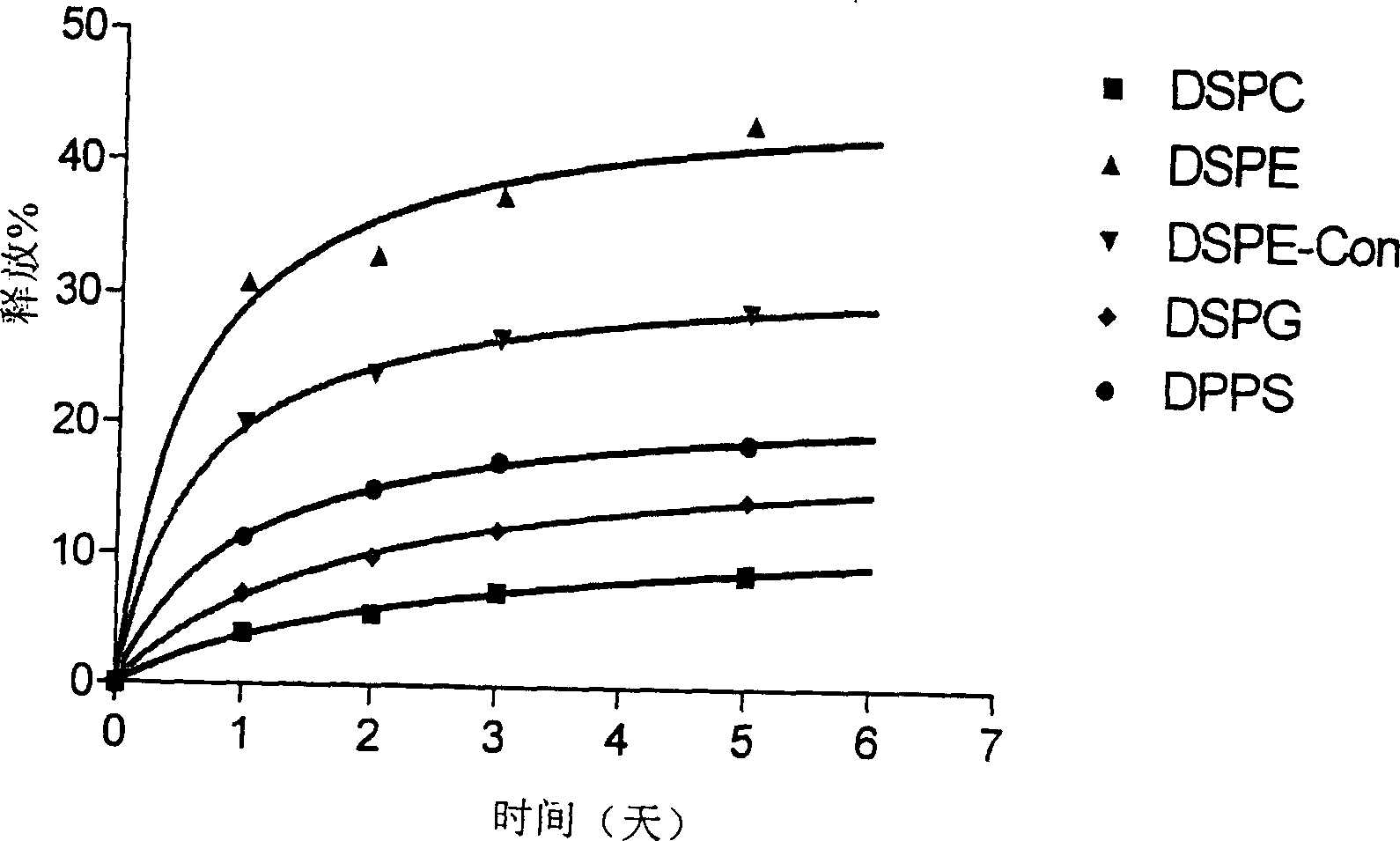
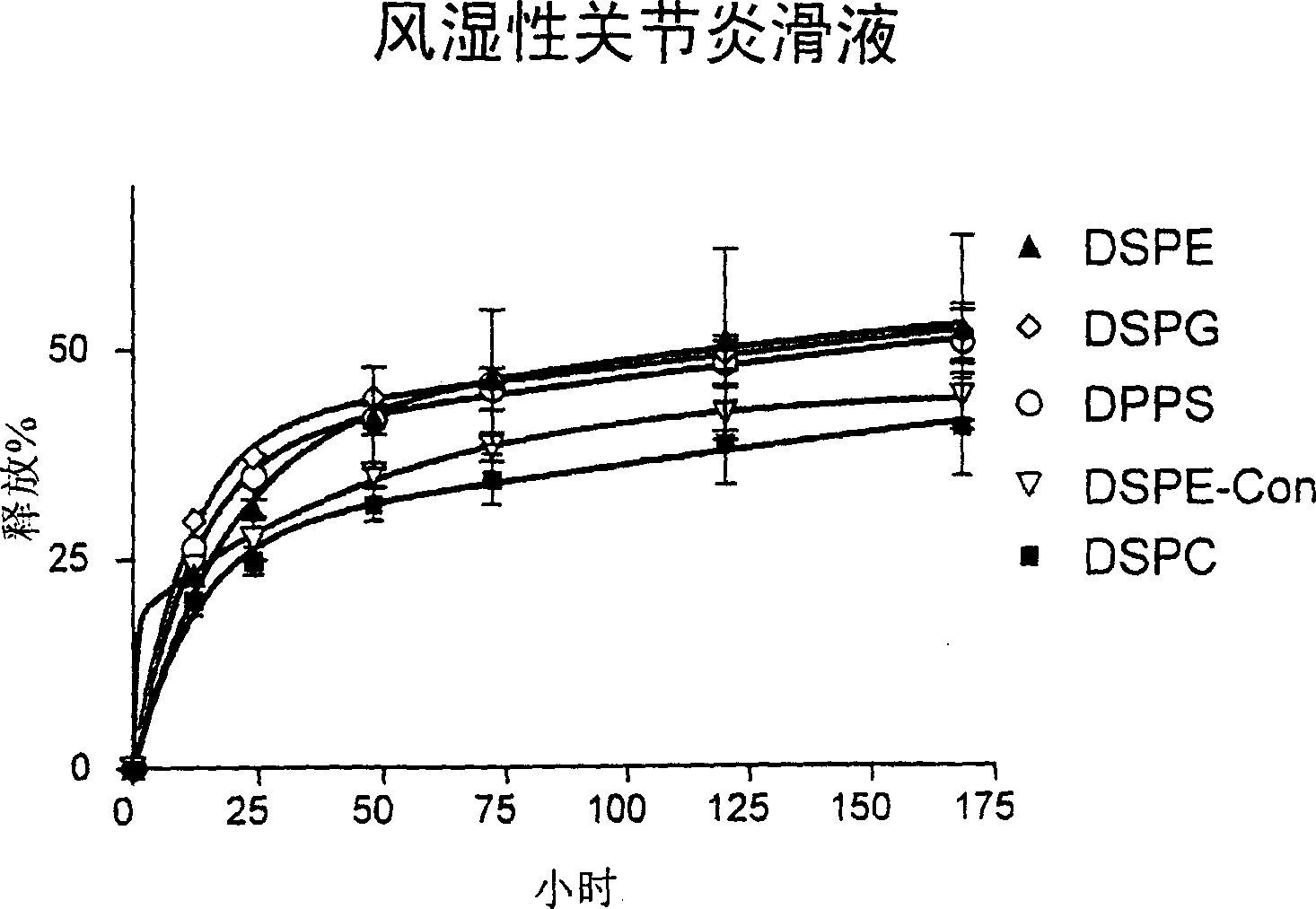
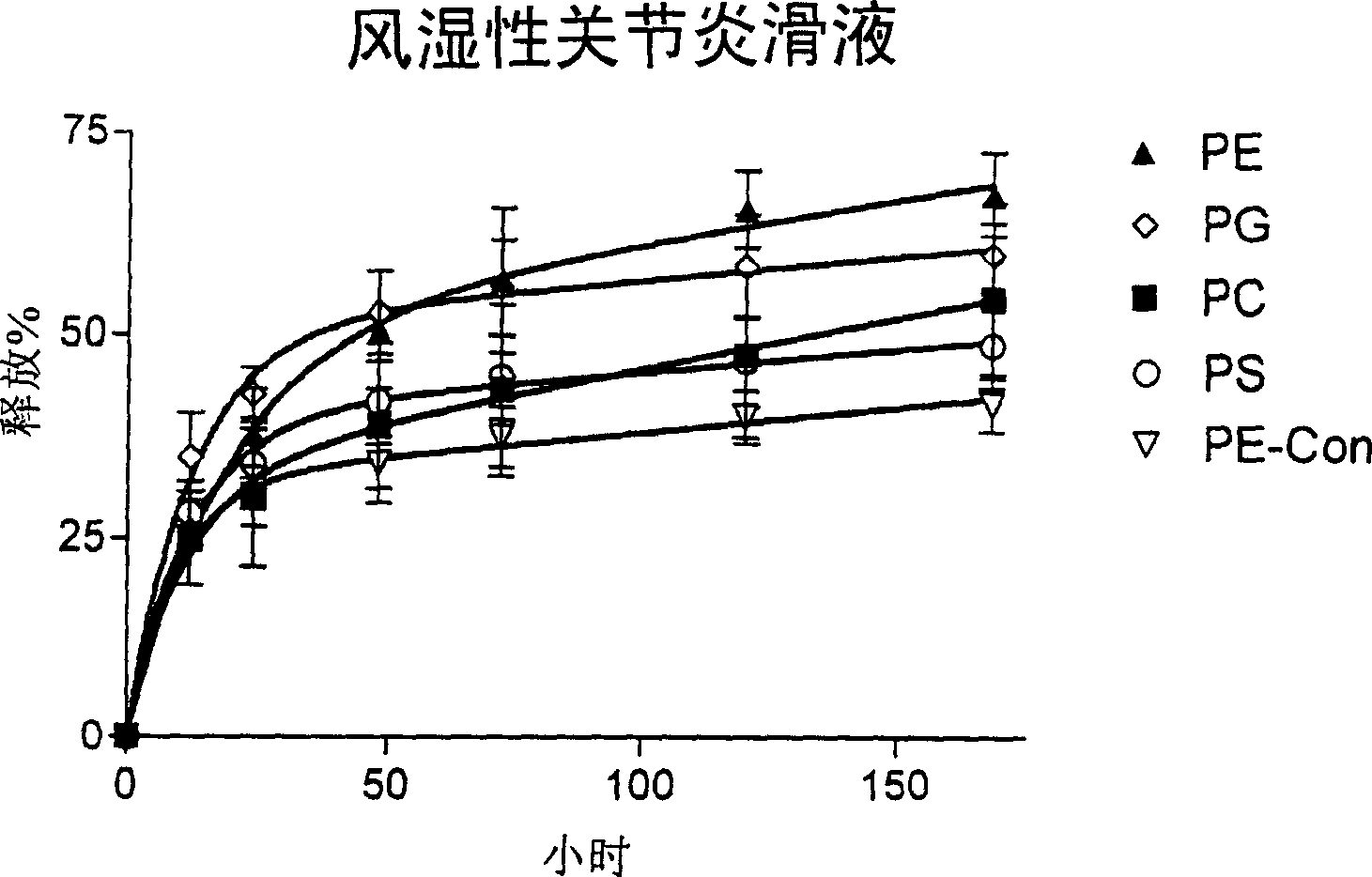
[0168] 100 μl of liposome-encapsulated GlcNAc prepared as described above was suspended in 3 ml of 0.1 M PBS (pH 7.4) in a 50 ml centrifuge tube. Liposomes were incubated at 37°C in a shaking incubator at 60 rpm. After various time points, liposomes were isolated by centrifugation at 20000 rpm for 15 minutes. GlcNAc released in the supernatant ( 3 H activity) was determined by liquid scintillation oscillator. The liposome pellet was resuspended in 3 ml of fresh PBS and incubated under the same conditions. Different types of liposomes [ 3 H] GlcNAc release percentage as a function of time is plotted in figure 1 .
[0169] figure 1 shows the in vitro release of different liposome formulations encapsulated in the presence of 0.1M PBS, pH 7.4 [ 3 H] Dynamic overview of GlcNAc. Determination of [ 3 H] Amount of GlcNAc, calculated as a percentage of initially encapsulated compound released.
[0170] figure 1 show[ 3 The...
Embodiment 3
[0176] In vivo retention of GlcNAc after intra-articular application
[0177] 50 μl of GlcNAc solution (unlabeled 2.69 mg / ml, labeled 5 μCi / ml, unlabeled molecule:labeled molecule 10000:1) was injected into the knee joints of six rats. The animals (2 animals per time point) were sacrificed 0, 30 minutes and 2 hours after application of the GlcNAc solution. Two additional rats were injected with saline as negative controls. All joint specimens were taken and homogenized with 5ml normal saline at 16000rpm for about 30 seconds. 100 μl of tissue homogenate was added to 500 μl of histolytic solution. The solution was incubated at 50°C for 16 hours, mixed with 30 μl of glacial acetic acid and 5 ml of liquid scintillation layer. Determined by liquid scintillation counting [ 3 H] GlcNAc activity.
[0178] Fourteen rats were used for each liposomal formulation. 50 [mu]l of liposomes (1.13 [mu]Ci / ml, GlcNAc concentration 0.61 mg / ml, unlabeled:labeled molecule 10000:1 prepared acco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com