Comonomer compsns. of prodn. of imide-contg. polyamino acids
A technology of comonomers and copolymers, applied in the direction of organic cleaning compositions, preparation of organic compounds, preparations for toiletry, etc., can solve problems that have not been successfully disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1. Preparation of a comonomer composition from a 1:1 equivalent solution of ammonium aspartate and monosodium aspartate, including air drying at 120°C.
[0101]In a 600 ml beaker, under magnetic stirring, an amount of 6.65 g of aspartic acid (0.05 mol, Mw133, Sigma Chemical, L isomer) was slurried in 50 ml of water. Add equal amount of NH 4 OH (32ml of 1:10 diluted concentrated ammonium hydroxide, 30% solution, 15.9M), converts aspartic acid into aspartic acid monoammonium in the solution. 8.65 g (0.05 mol) of easily soluble monosodium aspartate (monohydrate, Sigma Chemical) was added thereto. The solution was dried overnight at 120°C in air to form a solid light yellow but transparent glass-like disc.
[0102] The beaker containing the discs was quickly cooled by being partially immersed and rotated in a methanol bath (temperature close to -30°C) to which dry ice had been added, resulting in clean separation of the discs and the glass. Then the discs were pulverize...
Embodiment 2
[0104] Example 2. Preparation of a comonomer composition from a 1:1 equivalent solution of ammonium aspartate and monosodium aspartate, including vacuum drying at 120°C.
[0105] Following the method of Example 1, the difference is that a vacuum oven (VWR Scientific, Model 1430) set at 120° C. is used to complete the drying step under vacuum at a pressure of 50-100 mmHg.
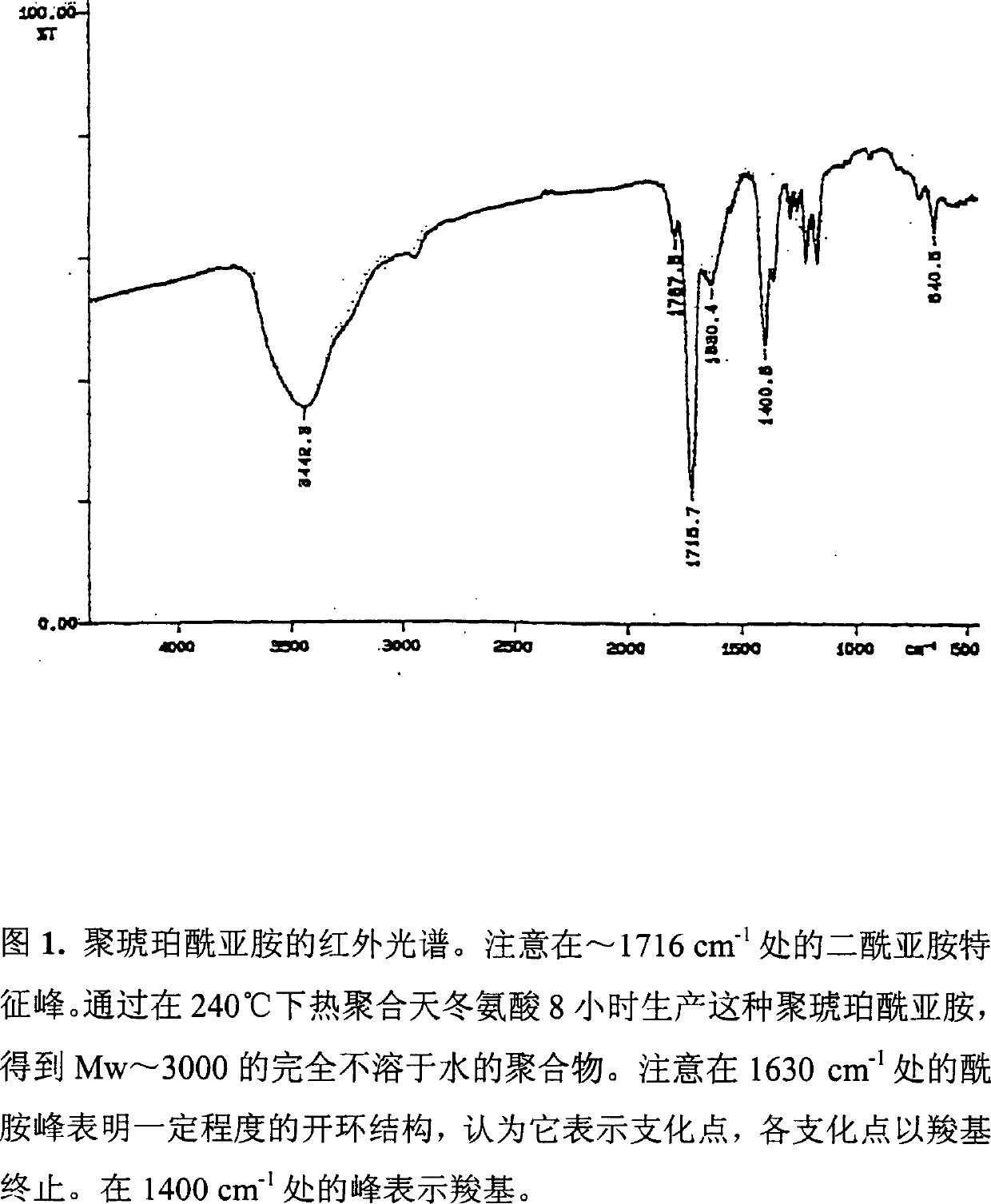
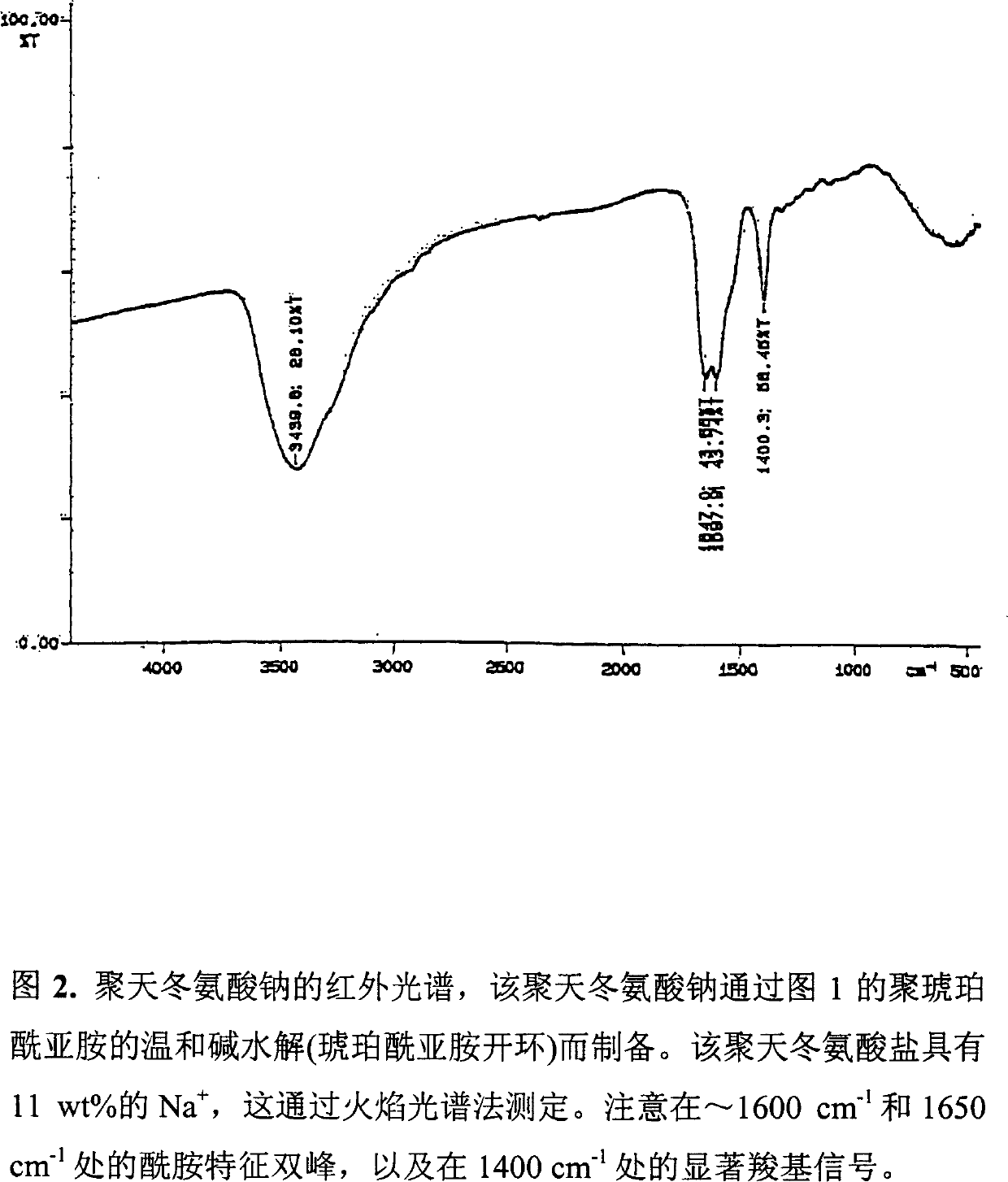
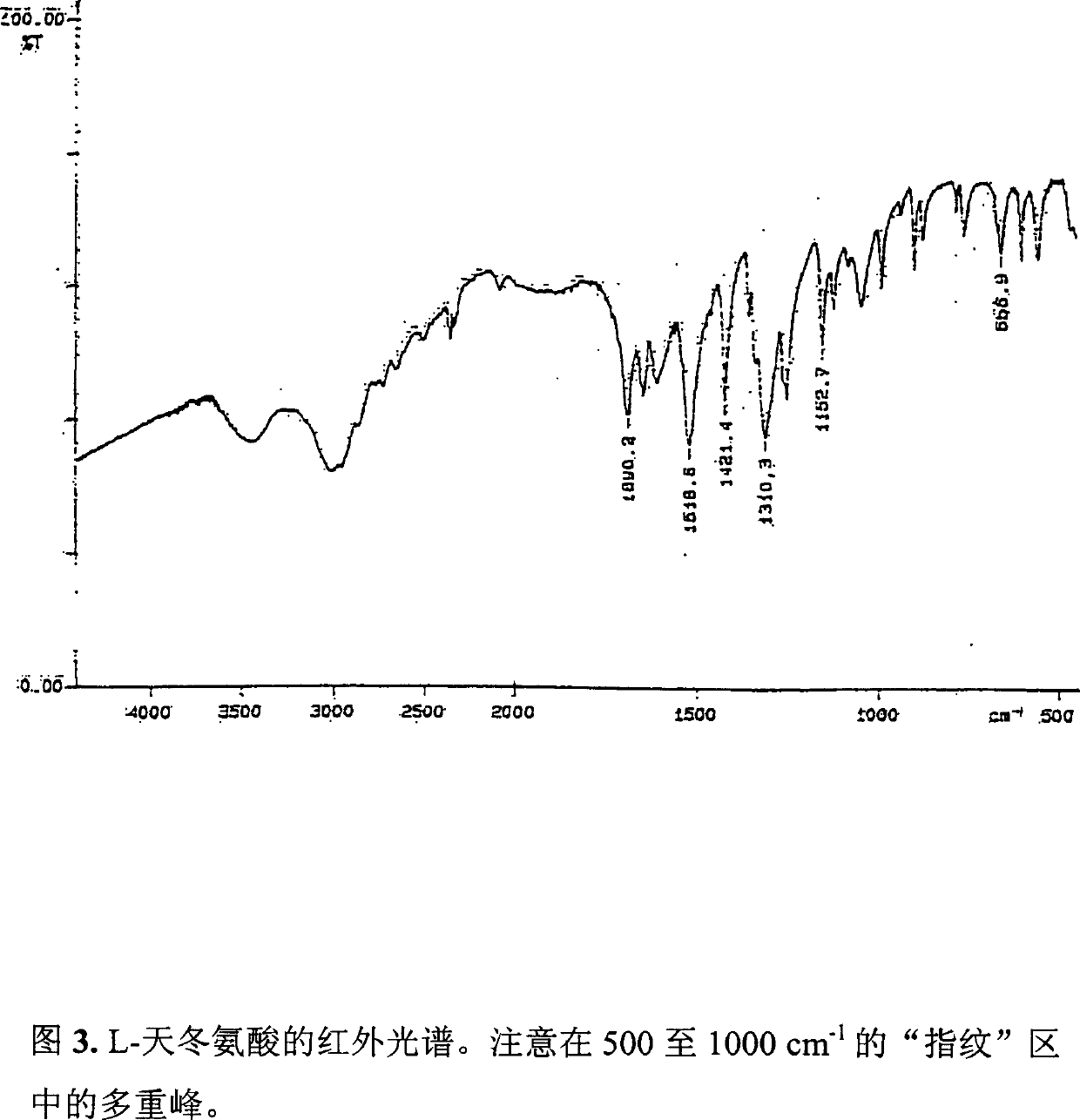
[0106] The resulting comonomer composition of sodium aspartate and aspartic acid was obtained with a yield of 15.37g, which was very close to the theoretical value (15.3g). This difference was also due to residual water and ammonium ion. In this case, the comonomer product is colorless, transparent and glassy (see the infrared spectrum of Figure 7).
Embodiment 3
[0107] Example 3. The copolymer was prepared from a 1:1 equivalent solution of ammonium aspartate and monosodium aspartate by adding 0.5 equivalent of sodium hydroxide to the ammonium aspartate solution, followed by vacuum drying at 120°C Monomer composition.
[0108] In a 600 ml beaker, under magnetic stirring, an amount of 6.65 g of aspartic acid (0.05 mol, Mw133, Sigma Chemical, L isomer) was slurried in 50 ml of water. Add equal amount of NH 4 OH (32ml of 1:10 diluted concentrated ammonium hydroxide, 30% solution, 15.9M), converts aspartic acid into aspartic acid monoammonium in the solution. 0.025 mol of sodium hydroxide was added thereto in the form of 2.5 ml of 10N sodium hydroxide. The solution was vacuum dried (50-100 mmHg) at 120°C overnight to form a solid, transparent glass-like disc.
[0109] The disc was processed as described in Example 1 to produce a solid, glassy, ground particulate comonomer composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com