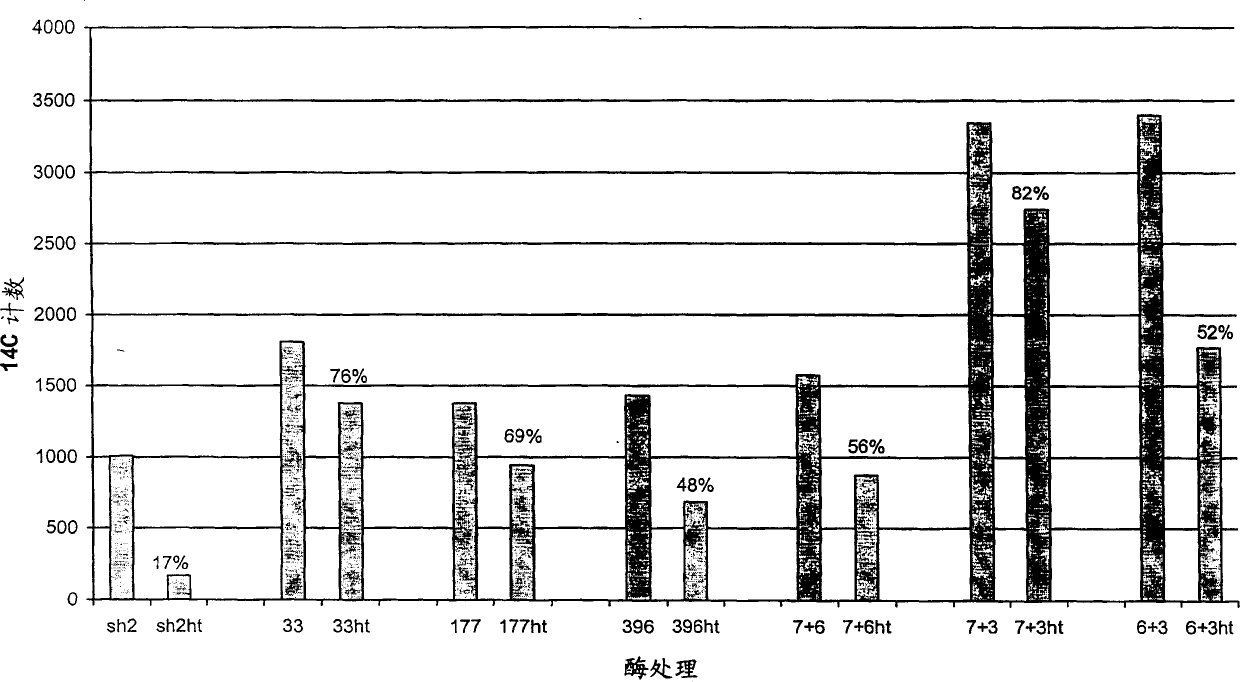
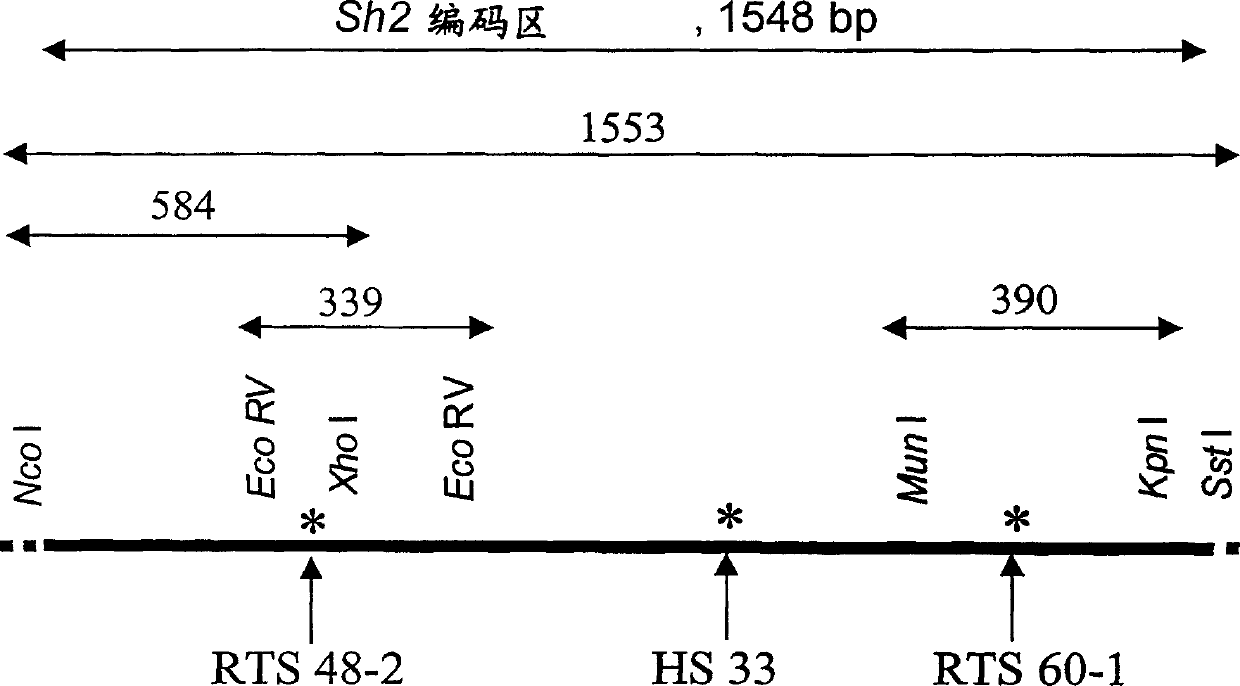
Heat stable mutants of starch biosynthesis enzymes
A technology of thermostability and mutant enzymes, applied in the fields of mutant preparation, biochemical equipment and methods, enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1——A test for corn endosperm ADP-glucose pyrophosphorylase containing multiple amino acid mutations
[0082] Expression of ADP-glucose pyrophosphorylase in maize endosperm.Inoculate 10 ml of Luria broth (75 g / mL spectinomycin and 50 g / mL kanamycin prime), and cultured overnight at 37°C with shaking at 220 rpm. These cultures were used to inoculate 250 mL of Luria broth (75 g / mL spectinomycin and 50 g / mL kanamycin). The culture was grown to OD at 37°C with shaking at 220rpm 600 = 0.55. Cultures were induced with 0.2 mM isopropyl-D-thiogalactopyranoside (IPTG) and .02 mg / mL nalidixic acid for 7 hours at room temperature with shaking at 220 rpm. Cells were collected by centrifugation at 3500 rpm for 10 min at 4°C. Resuspend the cell pellet in 800 L of extraction buffer. The above extraction buffer is 50mM HEPES, pH7.5, 5mM MgCl 2 , 5 mM EDTA, 20% sucrose and 30% ammonium sulfate. Add DTT (1mM), 50g / ml lysozyme, 1g / mL pepstatin, 1g / mL leupeptin, 1g / mL antipr...
Embodiment 2
[0103] Example 2 - Combination of thermostable mutations with Rev6
[0104] According to the invention, thermostable mutations may be combined with mutations associated with increased seed weight, such as the Rev6 mutation. The aim is to maintain the desired phosphate insensitivity profile of Rev6 while enhancing stability. Mutants containing Rev6 mutations in combination with thermostabilizing mutations can be constructed and validated as described herein. These "joint" mutants can be transformed into AC70R1-504 with the wild-type small subunit. Increased thermostability can be easily identified by positive glycogen staining on low glucose medium. Rev6 does not stain when grown on this medium. Initially, all combination mutants can be enzymatically screened for maintaining phosphate insensitivity, and then only combinations that maintain phosphate insensitivity are further analyzed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com