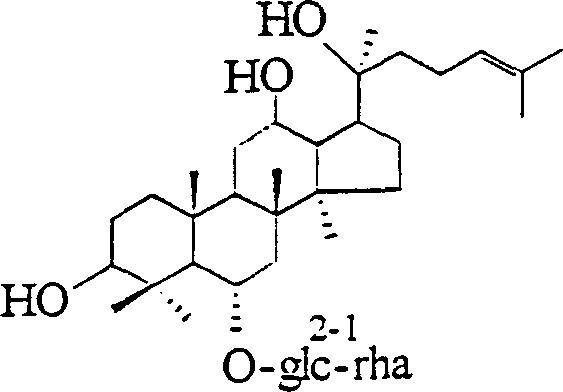
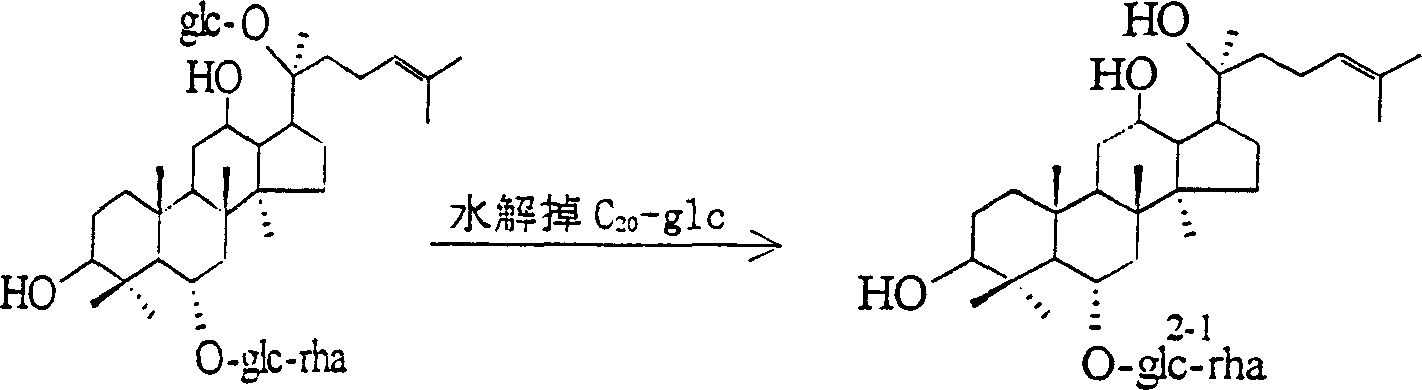Semi-synthesis method of 20(s)-ginsenoside Rg2
A ginsenoside, semi-synthetic technology, applied in steroids, organic chemistry and other directions, can solve the problems of low yield and complex synthesis method, and achieve the effect of simple method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Take 500mg of ginsenoside Re powder, dissolve it in 20% ethanol solution of 0.01M HCl, heat it for 2 hours in a water bath at 75°C, cool it, pass the solution through 717 anion exchange resin, remove acid, collect the effluent, and recover the solvent to dryness , to get ginsenoside Rg 2 crude product; then the crude product was taken and recrystallized repeatedly with methanol-ethyl acetate to obtain a pure product with a content greater than 98%, and the yield was 60%.
Embodiment 2
[0015] Take 500mg of ginsenoside Re powder, dissolve it in 30% ethanol solution of 0.05M HCl, heat it for 3 hours in a water bath at 80°C, cool it, pass the solution through 717 anion exchange resin, remove acid, collect the effluent, and recover the solvent to dryness , to get ginsenoside Rg 2 crude product; then, the ginsenoside Rg 2 The crude product was subjected to silica gel column chromatography, the eluent was chloroform-methanol-water (65:35:10), and the ginsenoside Rg 2 Part, recovery solvent gets ginsenoside Rg 2 Pure product, yield 63%.
Embodiment 3
[0017] Take 500 mg of ginsenoside Re powder, dissolve it in 30% ethanol solution of 0.09M HCl, heat it for 3 hours in a water bath at 60°C, cool it, pass the solution through 717 anion exchange resin, remove acid, collect the effluent, and recover the solvent to dryness , to obtain the crude product of ginsenoside Rg2; then, the crude product of ginsenoside Rg2 is subjected to silica gel column chromatography, the eluent is chloroform-methanol-water (65:35:10), the part of ginsenoside Rg2 is collected, and the solvent is recovered to obtain ginsenoside Rg2 pure product, yield 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com