Process for preparation of quinacridone series pigments
A technology of quinacridone and dihydroquinacridone, which is applied in quinacridone, chemical instruments and methods, azo dyes, etc., can solve the problems of many by-products, complex process, and large influence of pigment color light, etc., to achieve The effect of product quality assurance, simplification of process and improvement of production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
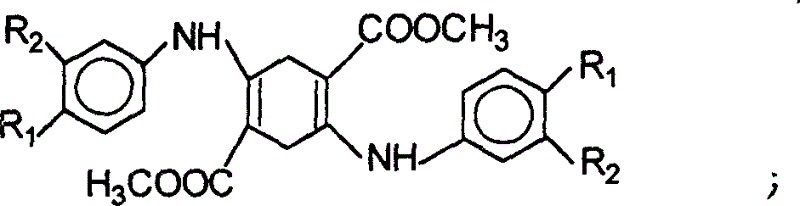
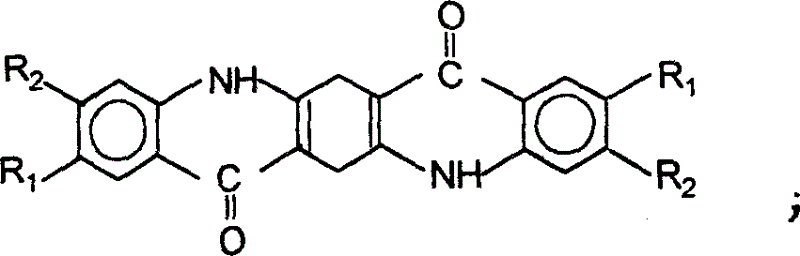
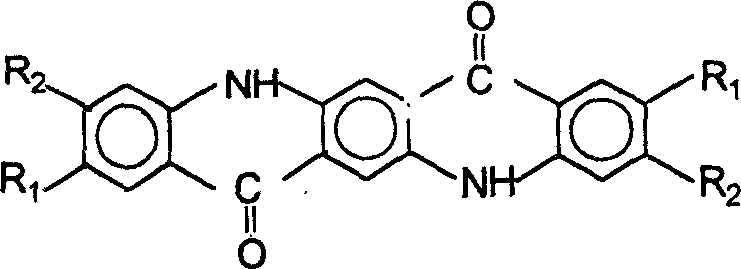
[0025] Preparation of Quinacridone
[0026] Step 1, condensation
[0027] Method 1: Dissolve 30 g of dimethyl succinyl succinate in 150 ml of ethanol, add 3 ml of concentrated hydrochloric acid and 26 g of aniline, and heat and reflux for 5 hours under nitrogen protection. Precipitate a large amount of orange-yellow solid, filter after cooling, wash the excess aniline with 0.5% dilute hydrochloric acid, wash with water until neutral, dry to obtain 1,4-(N-phenyl)-2,5-dicarboxylic acid dimethyl ester , the yield is 92%, and the content is 96-97%.
[0028] Method 2: Dissolve 30 g of dimethyl diacylsuccinate in 150 ml of ethanol, add 3 ml of concentrated hydrochloric acid and 30 g of p-methylaniline, and heat up and reflux for 5 hours under nitrogen protection. Precipitate a large amount of yellow-green solid, filter after cooling, wash the excess methylaniline with 0.5% dilute hydrochloric acid, wash with water until neutral, and dry to obtain 1,4-(N-p-tolylamino)-2,5-dicarboxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com