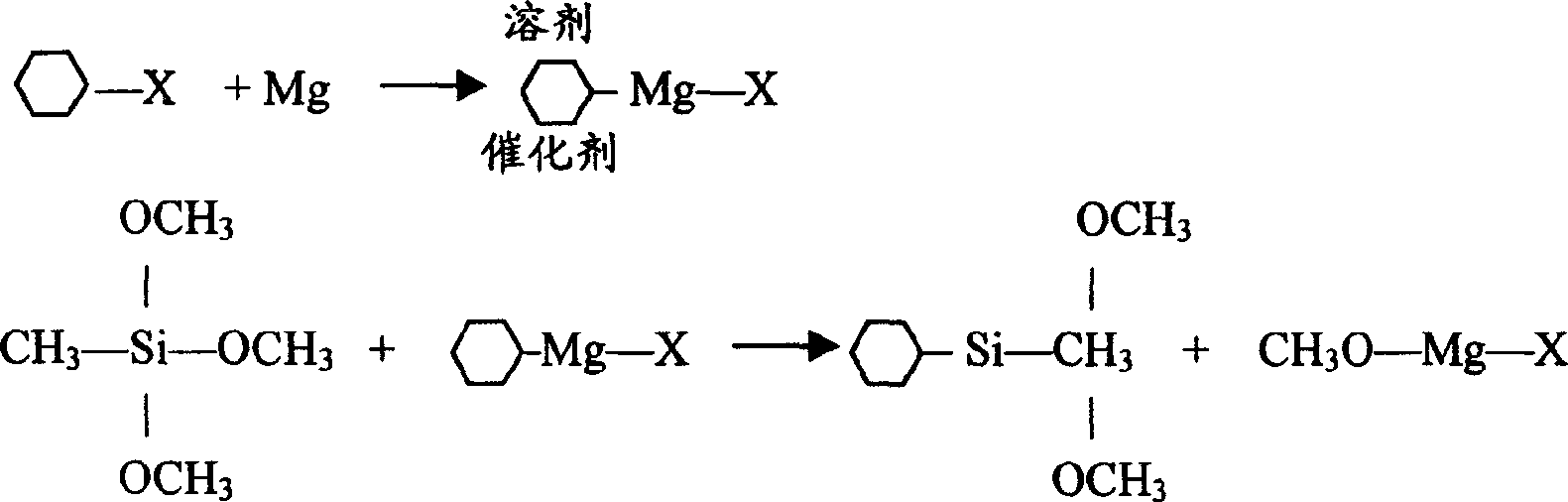
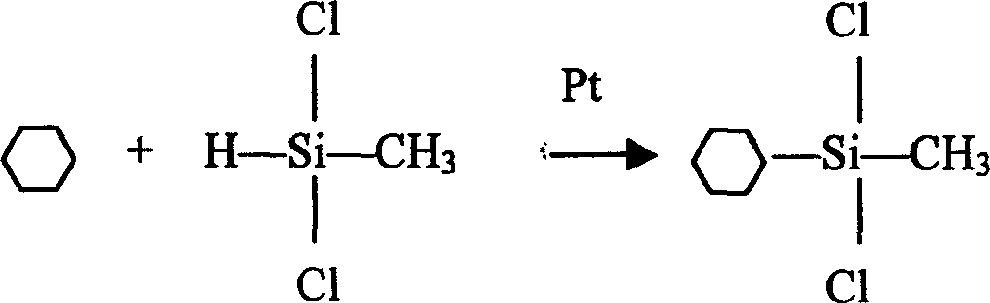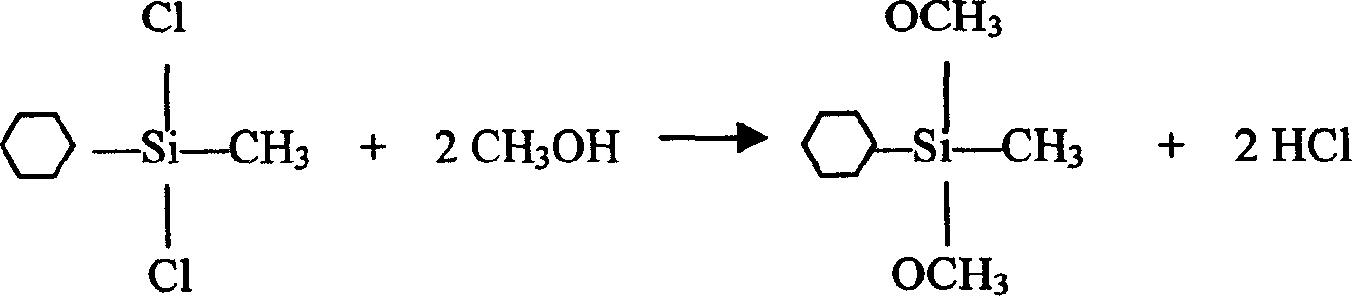One-step synthesizing cyclohexyl methyl dimethoxy silane without solvent
A technology of cyclohexylmethyldimethoxysilane and methyltrimethoxysilane, which is applied in the field of chemical raw materials, can solve problems such as harsh production and reaction conditions, high pressure requirements, and high risk, and achieve simple synthesis reaction operations Ease of operation, small equipment load and reasonable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] In a 250ml glass round bottom flask with reflux condenser, thermometer, stirrer, heater and balance feeder, under N 2 Add 12 grams of fresh magnesium powder, 56 grams of methyltrimethoxysilane, 5 grams of chlorocyclohexane and 0.2 gram of iodine under protection, and heat to keep the material under stable reflux. After reflux for 30 minutes, start to drop the remaining 41 grams of chlorocyclohexane. Cyclohexane was added completely in about 1 hour. After adding, keep warm for 30 minutes to end the reaction. The analysis results of the reaction solution showed that the conversion rate of cyclohexylmethyldimethoxysilane was 90.3% (calculated as silane), and the yield of cyclohexylmethyldimethoxysilane was 91.4%.
example 2
[0043] In a 500ml four-neck glass round bottom flask with a reflux condenser, thermometer, stirrer, heater and balance feeder, under N 2 Under protection, add 37 grams of fresh magnesium powder, 152 grams of methyltrimethoxysilane, 12 grams of chlorocyclohexane and 0.6 gram of potassium iodide, heat to keep the material under stable reflux, and after 30 minutes of reflux, start to add the remaining 138 grams of chlorine Substituting cyclohexane, use the time of about 1.5 hours to add all of them. After adding, keep warm for 30 minutes to end the reaction. The analysis results of the reaction solution showed that the conversion rate of cyclohexylmethyldimethoxysilane was 89.2% (calculated as silane), and the yield of cyclohexylmethyldimethoxysilane was 91.5%.
example 3
[0045] In a 500ml four-neck glass round bottom flask with a reflux condenser, a thermometer, a stirrer, a heater and a balance feeder, under N 2Under protection, add 37 grams of fresh magnesium powder, 152 grams of methyltrimethoxysilane, 12 grams of chlorocyclohexane and 0.6 gram of iodine and potassium iodide mixture (potassium iodide accounts for 20-60%), heat to keep the material stable reflux, reflux After 30 minutes, start to add the remaining 138 grams of chlorocyclohexane dropwise, and use the time of about 1.5 hours to add all of it. After adding, keep warm for 30 minutes to end the reaction. The analysis results of the reaction solution showed that the conversion rate of cyclohexylmethyldimethoxysilane was 91.5% (calculated as silane), and the yield of cyclohexylmethyldimethoxysilane was 93.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com