Radical-metal complex type molecular ferromagnet and preparation method
A technology of metal complexes and free radicals, which is applied in the field of free radical-metal complex molecular ferromagnets and preparation, can solve the problems of difficult synthesis of three free radicals and low yield, and achieve easy composite processing and small size , the effect of light weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The synthesis of embodiment 1 free radical NITPhCl:
[0021] (1). Synthesis of 2,3-dimethyl-2,3-dinitrobutane (I) [L.W.Seigle, J.Org.Chem., 1940, 5, 100; P.Sayre, J.Am. Chem.Soc., 1955,71,6689] the NaOH solution of 45g (0.5mol) 2-nitropropane and 84ml 6N is added in the 500mL three-neck flask, ice-water bath cooling, mechanical stirring 0.5 hour, then slowly drip 40g ( 0.25mol) of liquid bromine, and then add 165ml of absolute ethanol, and continue to stir for 0.5 hours. It was then refluxed at 85°C for 3 hours, naturally cooled to room temperature, suction filtered, washed several times with distilled water, and dried to obtain 29 g of white flaky crystals with a yield of 65% and a melting point of 129-130°C.
[0022] Reaction formula:
[0023]
[0024] (2) Synthesis of 2,3-dimethyl-2,3-dihydroxyaminobutane (II) [M.Lamchem, J.Chem.Soc., 1966, 2300]
[0025] 10g NH 4 Dissolve Cl in 200ml of 1:1 ethanol-water solution, add 17.5g of product (I) under an ice-water b...
Embodiment 2
[0036] Example 2 manganese hexafluoroacetylacetonate Mn(hfac) 2 2H 2 Synthesis of O (P.A.Cotton J.Am.Chem.Soc., 1960, 82, 2979):
[0037] Weigh 4.8 grams of Mn(Ac) 2 2H 2 O, dissolved in 300ml 50% water-ethanol solution, added 200ml (10g) hfac, stirred, the solution turned yellow, evaporated, dried to obtain bright yellow flaky crystals. Yield: 68%.
Embodiment 3
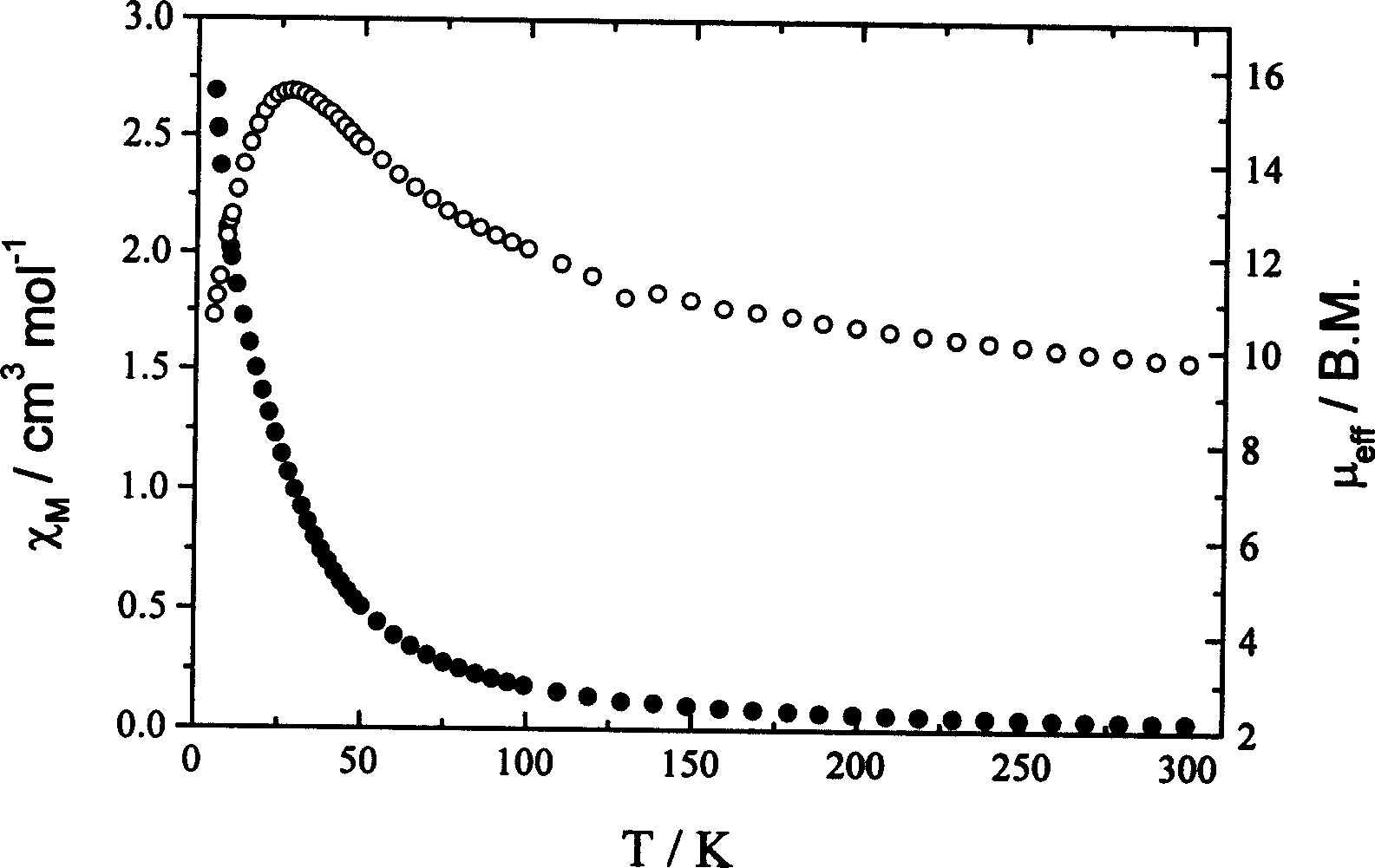
[0038] Example 3 Synthesis and characterization of free radical-metal complexes:
[0039] (1) Synthesis: Add the Mn(hfac) synthesized above in the reaction vessel 2 2H 2 O 505mg, then add 100ml of anhydrous n-heptane, heat to boiling, cool down to 70°C after dissolving, add 50ml of anhydrous n-heptane solution containing 268mg NITPhCl free radical; after reacting for 10 minutes, cool down to room temperature, filter, and After standing for one week, green crystals were precipitated, and the yield was 51% (436 g).
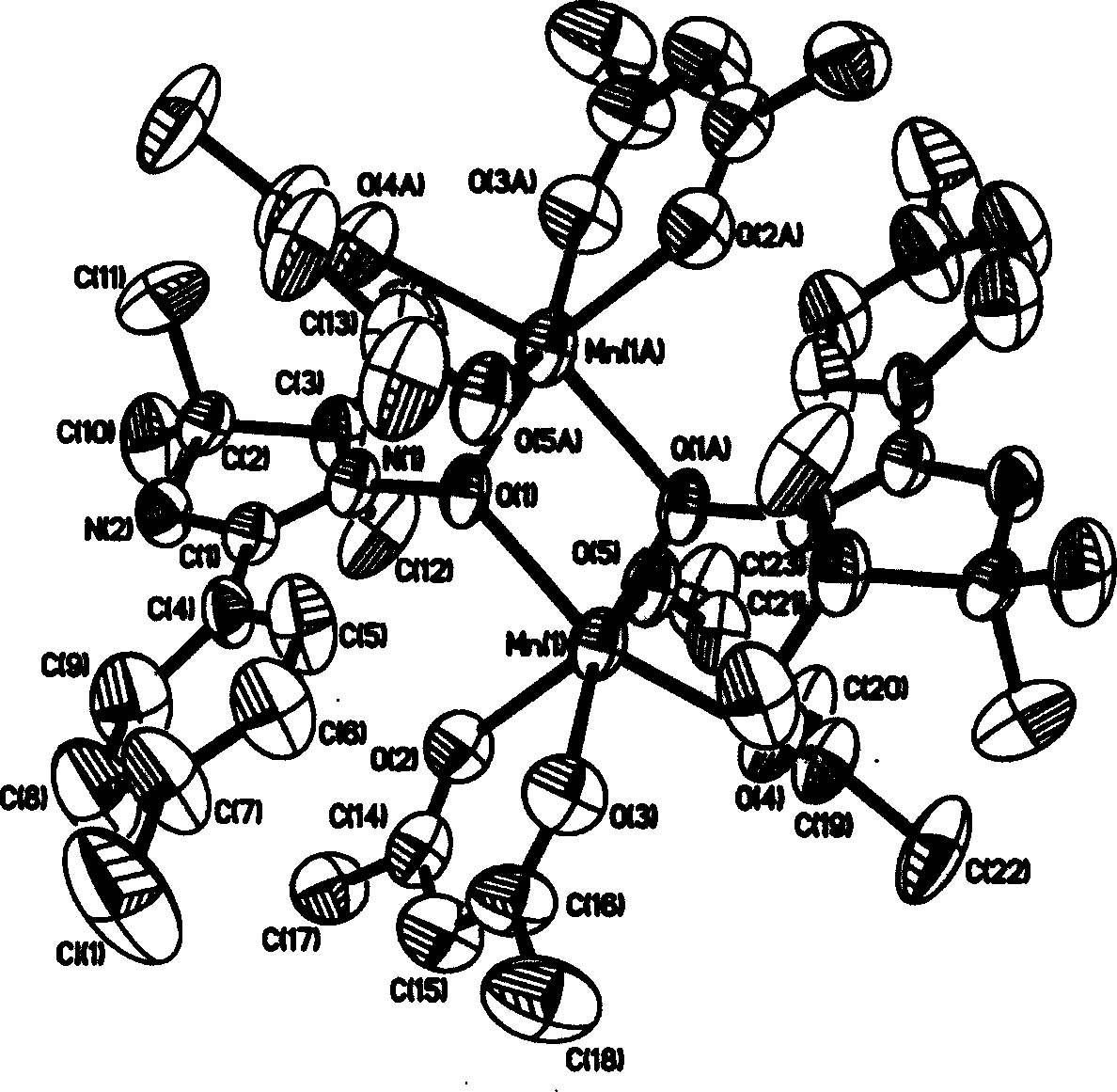
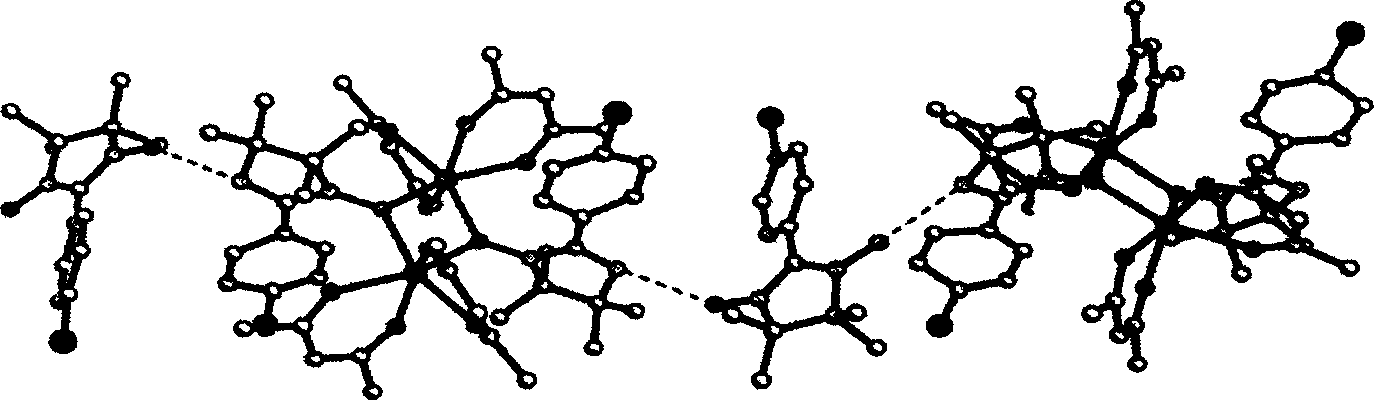
[0040] (2). Determination of the structure of the complex
[0041] Select a green crystal with a size of about 0.10×0.20×0.25mm, use graphite monochromatized Mokα rays (λ=0.71073 ) as incident radiation on a BRUKER SMART 1000 X-ray diffractometer, and measure the temperature at 298 (2) K, the unit cell parameters were corrected by the least square method from 12911 diffraction points, and the crystal structure was solved by the SHELXL-97 direct method from the di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com