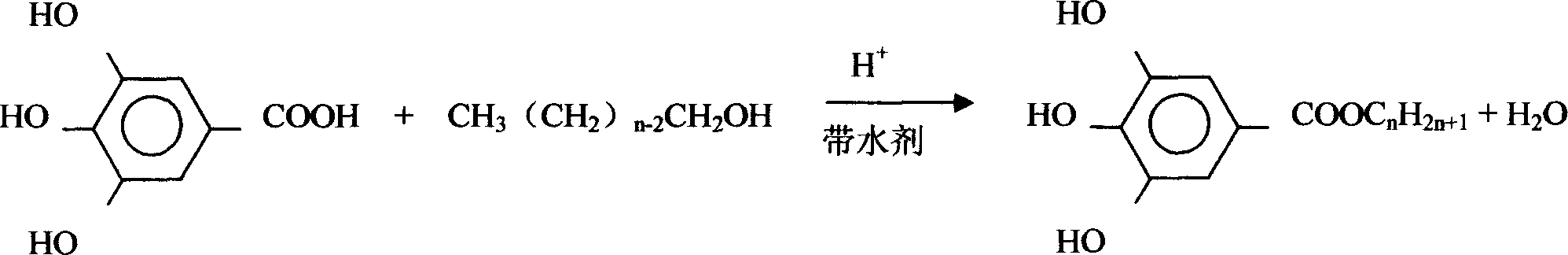Preparation method of gallic acid high grade alxoxide ester
A technology for acid higher alkanol ester and gallic acid, which is applied in the field of preparation of higher gallic acid alkanol ester, can solve the problems of difficult control of process conditions, difficult regeneration and recycling, difficulty in industrial application, etc. Programmatic, the effect of shortened response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The reactor with stirring, thermometer and heating mantle is connected with condenser and alumina microporous ceramic tube in turn.
[0019] Put 96g of gallic acid, 88g of n-octanol, 28g of catalyst dodecylbenzenesulfonic acid and an appropriate amount of water-bearing solvent 1,4-dioxane into the reactor, stir and heat, and keep the temperature in the reactor at 108-110°C. reaction for 8 hours. During this process, the gaseous 1,4-dioxane in the reactor will take the water generated by the reaction from the top of the reactor in the form of steam, condense into a liquid phase through the condenser, and absorb the water through the alumina microporous ceramic tube Return to the reactor for recycling. After the reaction was finished, the reactant was distilled under reduced pressure to recover the solvent, then heated to dissolve with toluene, and the solution was recrystallized to obtain 148 g of octyl gallate, with a yield of 94%. The melting point of the product is ...
Embodiment 2
[0021] The device is the same as in Example 1, put into the reactor 96g of gallic acid, 215g of stearyl alcohol, 15g of catalyst p-toluenesulfonic acid, and an appropriate amount of solvent 1,4-dioxane, stir and heat, and keep the temperature in the device at 115-120°C. reaction for 14 hours. After the solvent was recovered from the reaction mixture, the crude product was dissolved in xylene and recrystallized to obtain 373 g of stearyl gallate (92% yield), with a melting point of 101-102°C. As determined by mass spectrometry, the molecular weight of the product was 422.
Embodiment 3
[0022] Example 3 The yields of various products of higher alkanol gallate prepared by the method of the present invention are shown in the following table.
[0023] sequence
[0024] Acids Alcohols Catalyst Water with water Solvent Product Yield%
[0025] No
[0026] 1 Gallic acid n-octanol Dodecylbenzenesulfonic acid 1,4-dioxane Octyl gallate 94
[0027] 2 Gallic acid Lauryl alcohol p-toluenesulfonic acid 1,4-dioxane Lauryl gallate 97
[0028] 3 gallic acid myristyl alcohol p-toluenesulfonic acid 1,4-dioxane tetradecyl gallate 94
[0029] 4 gallic acid cetyl alcohol p-toluenesulfonic acid 1,4-dioxane cetyl gallate 91
[0030] 5 gallic acid stearyl alcohol p-toluenesulfonic acid 1,4-dioxane octadecyl gallate 92
[0031] 6 gallic acid eicosanol p-toluenesulfonic acid 1,4-dioxane eicosyl gallate 93
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com